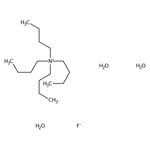
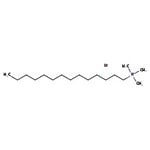
Search Thermo Fisher Scientific

Quaternary ammonium salts
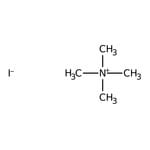
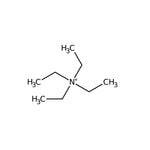
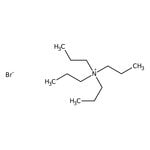
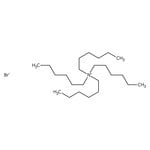
Quaternary ammonium salts or compounds, also known as quats, are organonitrogen compounds that contain a positively charged nitrogen atom with four single bonds to carbon atoms or other functional groups.
Products (280)
Learn More (0)
Documents & Support
(0)280 Products
Filter
Product | Brand | Quantity | Boiling Point | Size | Price | |
|---|---|---|---|---|---|---|
| — | 100 mL | — | Each | |||
| — | 100 g | — | Each | |||
| — | 100 mL | — | Each | |||
| — | 25 g | — | Bottle | |||
| — | 25 g | — | Bottle | |||
| — | 50 g | — | Each | |||
| — | 100 g | — | Each | |||
| — | 50 g | — | Each | |||
| — | 50 g | — | Each | |||
| — | 500 g | — | Each | |||
| — | 100 g | — | Each | |||
| — | 2500 g | — | Each | |||
| — | 100 mL | — | Each | |||
| — | 1 L | — | Each | |||
| — | 250 mL | — | Each | |||
| — | 5 g | — | Each | |||
| — | 25 g | — | Each | |||
| — | 50 g | — | Each | |||
| — | 10 g | — | Each | |||
| — | 2 g | — | Each | |||
| — | 0.5 g | — | Each | |||
| — | 250 g | — | Each | |||
| — | 50 g | — | Each | |||
| — | 50 g | — | Each | |||
| — | 10 g | — | Each | |||
| — | 500 g | — | Each | |||
| — | 100 g | — | Each | |||
| — | 1 L | ∼102°C | Each | |||
| — | 250 mL | ∼102°C | Each | |||
| — | 25 mL | ∼102°C | Each |
Tetrabutylammonium phosphate monobasic, 1M solution in ethanol, AcroSeal™ Thermo Scientific Chemicals
This Thermo Scientific Chemicals brand product was originally part of the Acros Organics product portfolio. Some documentation and label information may refer to the legacy brand. The original Acros Organics product / item code or SKU reference has not changed as a part of the brand transition to...
Tetramethylammonium iodide, 99% Thermo Scientific Chemicals
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo...
Tetrabutylammonium phosphate monobasic, 1M solution in ethanol, AcroSeal™ Thermo Scientific Chemicals
This Thermo Scientific Chemicals brand product was originally part of the Acros Organics product portfolio. Some documentation and label information may refer to the legacy brand. The original Acros Organics product / item code or SKU reference has not changed as a part of the brand transition to...
(2-Chloroethyl)trimethylammonium chloride, 98% Thermo Scientific Chemicals
This Thermo Scientific Chemicals brand product was originally part of the Acros Organics product portfolio. Some documentation and label information may refer to the legacy brand. The original Acros Organics product / item code or SKU reference has not changed as a part of the brand transition to...
(2-Chloroethyl)trimethylammonium chloride, 98% Thermo Scientific Chemicals
This Thermo Scientific Chemicals brand product was originally part of the Acros Organics product portfolio. Some documentation and label information may refer to the legacy brand. The original Acros Organics product / item code or SKU reference has not changed as a part of the brand transition to...
Tetraethylammonium iodide, 98+% Thermo Scientific Chemicals
It has been used as the source of tetraethylammonium ions in pharmacological and physiological studies, but is also used in organic chemical synthesis. This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio.
Tetra-n-propylammonium bromide, 98% Thermo Scientific Chemicals
Tetra-n-propylammonium bromide, is used as surface-active agents, Solvents, Intermediates, Active Ingredient for Conditioners, Antistatic Agent, Detergent Sanitisers, Softner for textiles and paper products, Phase Transfer Catalyst, Antimicrobials, Disinfection Agents And Sanitizers, Slimicidal...
Tetra-n-hexylammonium bromide, 98% Thermo Scientific Chemicals
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo...
(1-Dodecyl)trimethylammonium bromide, 99% Thermo Scientific Chemicals
(1-Dodecyl)trimethylammonium bromide is used as a surfactant, as an emulsifier of rubber and asphalt and as an anti-static agent of synthetic fiber. It acts as an ionic surfactant and useful in the preparation of gold nanopartilces along with sodium dodecylsulfate.
Tetra-n-butylammonium bromide, 98+% Thermo Scientific Chemicals
Phase transfer catalyst Tetra-n-butylammonium bromide is used as a phase transfer catalyst for the preparation of lactones by cyclization of potassium salt of μ-bromocarboxylic acids and beta-lactones from beta-bromoacid by cyclization.
Tetra-n-butylammonium bromide, 98+% Thermo Scientific Chemicals
Phase transfer catalyst Tetra-n-butylammonium bromide is used as a phase transfer catalyst for the preparation of lactones by cyclization of potassium salt of μ-bromocarboxylic acids and beta-lactones from beta-bromoacid by cyclization.
Tetra-n-butylammonium bromide, 98+% Thermo Scientific Chemicals
Phase transfer catalyst Tetra-n-butylammonium bromide is used as a phase transfer catalyst for the preparation of lactones by cyclization of potassium salt of μ-bromocarboxylic acids and beta-lactones from beta-bromoacid by cyclization.
Tetra-n-butylammonium hydroxide, 1.0M aq. soln. Thermo Scientific Chemicals
Tetra-n-butylammonium hydroxide is used in biological studies for the detection of counter-anion effect on antimicrobial activity of tetrabutylammonium salts, intermediates, Plating agents and surface treating agents, laboratory chemicals.
Tetramethylammonium hydroxide, 1.0 M aq. soln., ACS Thermo Scientific Chemicals
Tetramethylammonium hydroxide is used as anisotropic etching of silicon, as a basic solvent in the development of acidic photo resist in the photolithography process, and is highly effective in stripping photo resist, and is used as a surfactant in the synthesis of ferrofluid, to inhibit...
Tetramethylammonium hydroxide, 1.0 M aq. soln., ACS Thermo Scientific Chemicals
Tetramethylammonium hydroxide is used as anisotropic etching of silicon, as a basic solvent in the development of acidic photo resist in the photolithography process, and is highly effective in stripping photo resist, and is used as a surfactant in the synthesis of ferrofluid, to inhibit...
N-Dodecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate Thermo Scientific Chemicals
For protein solubilization This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand.
(1-Hexadecyl)trimethylammonium chloride, 96% Thermo Scientific Chemicals
(1-Hexadecyl)trimethylammonium chloride is used as a topical antiseptic and surfactant. It is used in hair care formulations, cream rinse ingredient, hair conditioners and shampoos as a conditioning agent.
Tetra-n-butylammonium phosphate, 99+% Thermo Scientific Chemicals
Tetra-n-butylammonium phosphate is used as a counter-ion in ion-pair reagent in high-performance liquid chromatography for the simultaneous determination of chlorpheniramine and maleate of water-soluble vitamins. It acts as a phase transfer catalyst.
Tetra-n-butylammonium phosphate, 99+% Thermo Scientific Chemicals
Tetra-n-butylammonium phosphate is used as a counter-ion in ion-pair reagent in high-performance liquid chromatography for the simultaneous determination of chlorpheniramine and maleate of water-soluble vitamins. It acts as a phase transfer catalyst.
Tetra-n-butylammonium phosphate, 99+% Thermo Scientific Chemicals
Tetra-n-butylammonium phosphate is used as a counter-ion in ion-pair reagent in high-performance liquid chromatography for the simultaneous determination of chlorpheniramine and maleate of water-soluble vitamins. It acts as a phase transfer catalyst.
Tetra-n-butylammonium phosphate, 99+% Thermo Scientific Chemicals
Tetra-n-butylammonium phosphate is used as a counter-ion in ion-pair reagent in high-performance liquid chromatography for the simultaneous determination of chlorpheniramine and maleate of water-soluble vitamins. It acts as a phase transfer catalyst.
Tetra-n-butylammonium hydroxide, 55% w/w aq. soln. Thermo Scientific Chemicals
As a reagent for ion-pair extractionsTetra-n-butylammonium hydroxide, 55% w/w aq. solution is used as a phase-transfer catalyst in organic synthesis especially for alkylation and condensation reactions. It is used as a curing accelerator for epoxy resins.
Tetra-n-butylammonium hydroxide, 55% w/w aq. soln. Thermo Scientific Chemicals
As a reagent for ion-pair extractionsTetra-n-butylammonium hydroxide, 55% w/w aq. solution is used as a phase-transfer catalyst in organic synthesis especially for alkylation and condensation reactions. It is used as a curing accelerator for epoxy resins.
Tetra-n-butylammonium fluoride trihydrate, 98% Thermo Scientific Chemicals
Tetra-n-butylammonium fluoride trihydrate is used as a phase transfer catalyst, as a mild base and as a source of fluoride ion in organic solvents. It is also used as a deprotecting agent to remove silyl ether protecting groups.
Tetra-n-butylammonium fluoride trihydrate, 98% Thermo Scientific Chemicals
Tetra-n-butylammonium fluoride trihydrate is used as a phase transfer catalyst, as a mild base and as a source of fluoride ion in organic solvents. It is also used as a deprotecting agent to remove silyl ether protecting groups.
(1-Tetradecyl)trimethylammonium bromide, 98% Thermo Scientific Chemicals
Myristyltrimethylammonium has been used in a study to assess a surfactant-controlled synthetic method to obtain a nanophase of mesoporous ceria-zirconia solid solution containing cationic defects in the crystalline structure.
(1-Tetradecyl)trimethylammonium bromide, 98% Thermo Scientific Chemicals
Myristyltrimethylammonium has been used in a study to assess a surfactant-controlled synthetic method to obtain a nanophase of mesoporous ceria-zirconia solid solution containing cationic defects in the crystalline structure.
Tetramethylammonium hydroxide, 25% w/w aq. soln., Thermo Scientific Chemicals Thermo Scientific Chemicals
Tetramethylammonium hydroxide is used to produce tetramethyl-ammonium azide. It is used as an anisotropic etchant of silicon, as a basic solvent in the development of acidic photoresist in photolithography process, as a surfactant in the synthesis of ferrofluid and as a polarographic reagent.
Tetramethylammonium hydroxide, 25% w/w aq. soln., Thermo Scientific™ Thermo Scientific Chemicals
Tetramethylammonium hydroxide is used to produce tetramethyl-ammonium azide. It is used as an anisotropic etchant of silicon, as a basic solvent in the development of acidic photoresist in photolithography process, as a surfactant in the synthesis of ferrofluid and as a polarographic reagent.
Tetramethylammonium hydroxide, 25% w/w aq. soln., Thermo Scientific Chemicals Thermo Scientific Chemicals
Tetramethylammonium hydroxide is used to produce tetramethyl-ammonium azide. It is used as an anisotropic etchant of silicon, as a basic solvent in the development of acidic photoresist in photolithography process, as a surfactant in the synthesis of ferrofluid and as a polarographic reagent.