Search Thermo Fisher Scientific
Thermo Scientific Chemicals
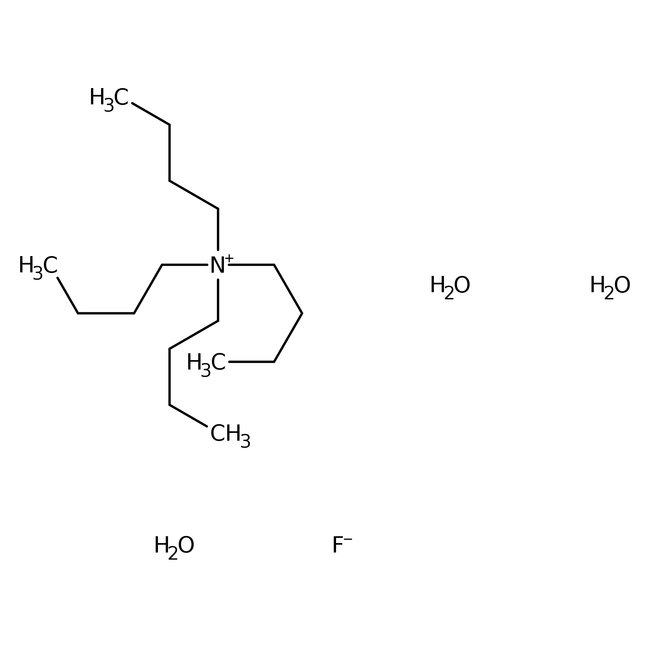
Tetra-n-butylammonium fluoride trihydrate, 98%, Thermo Scientific Chemicals
CAS: 87749-50-6 | C16H42FNO3 | 315.514 g/mol
Catalog number ALFL13303.18
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
50 g
Chemical Identifiers
CAS7079-48-3
IUPAC Name4-fluoro-2-methylbenzene-1-sulfonyl chloride
Molecular FormulaC7H6ClFO2S
InChI KeyXLPGWKNCWMFHOD-UHFFFAOYSA-N
SMILESCC1=C(C=CC(F)=C1)S(Cl)(=O)=O
View more
Specifications Specification Sheet
Specification Sheet
Refractive Index1.5340-1.5390 @ 20?C
Appearance (Color)Clear colorless to yellow
Assay (GC)≥96.0%
FormLiquid
Tetra-n-butylammonium fluoride trihydrate is used as a phase transfer catalyst, as a mild base and as a source of fluoride ion in organic solvents. It is also used as a deprotecting agent to remove silyl ether protecting groups. It acts as a reactant for the preparation of deprotecting agents, in preparation of cellulose derivatives, lipophilic peptides for DNA transfections and Dehydrobromination reactions. It plays an important role in the convertion of O-silylated enolates in to carbonyls in the presence of dimethyl sulfoxide.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Tetra-n-butylammonium fluoride trihydrate is used as a phase transfer catalyst, as a mild base and as a source of fluoride ion in organic solvents. It is also used as a deprotecting agent to remove silyl ether protecting groups. It acts as a reactant for the preparation of deprotecting agents, in preparation of cellulose derivatives, lipophilic peptides for DNA transfections and Dehydrobromination reactions. It plays an important role in the convertion of O-silylated enolates in to carbonyls in the presence of dimethyl sulfoxide.
Solubility
Soluble in water, terahydrofuran, dimethyl sulfoxide and acetonitrile.
Notes
Hygroscopic. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents.
Tetra-n-butylammonium fluoride trihydrate is used as a phase transfer catalyst, as a mild base and as a source of fluoride ion in organic solvents. It is also used as a deprotecting agent to remove silyl ether protecting groups. It acts as a reactant for the preparation of deprotecting agents, in preparation of cellulose derivatives, lipophilic peptides for DNA transfections and Dehydrobromination reactions. It plays an important role in the convertion of O-silylated enolates in to carbonyls in the presence of dimethyl sulfoxide.
Solubility
Soluble in water, terahydrofuran, dimethyl sulfoxide and acetonitrile.
Notes
Hygroscopic. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Huang, S. H.; Liu, C. H.; Yang, C. M. Efficient ligand-free Hiyama cross-coupling reaction catalyzed by functionalized SBA-15-supported Pd nanoparticles. Green Chem. 2014, 16 (5), 2706-2712.
- Liang, X.; Kozlovskaya, V.; Cox, C. P.; Wang, Y.; Saeed, M.; Kharlampieva, E. Synthesis and self-assembly of thermosensitive double-hydrophilic poly(N-vinylcaprolactam)-b-poly(N-vinyl-2-pyrrolidone) diblock copolymers. J. Polym. Sci., Part A: Polym. Chem. 2014, 52 (19), 2725-2737.