Search Thermo Fisher Scientific

Organotin
Organotin compounds are based on tin with hydrocarbon substituents and commonly have an oxidation state of tin(IV). Available in various chemical compositions, these compounds have multiple industrial and research applications.
Products (5)
Learn More (0)
Documents & Support
(0)5 Products
Filter
Product | Boiling Point | Molecular Weight (g/mol) | Percent Purity | Size | Price | |
|---|---|---|---|---|---|---|
| >204°C (12 mmHg) | — | 95% | Each | |||
| >204°C (12 mmHg) | — | 95% | Each | |||
| >204°C (12 mmHg) | — | 95% | Each | |||
| — | 271.805 | 97% | Each | |||
| — | 271.805 | 97% | Each |
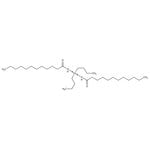
Di-n-butyltin dilaurate, 95% Thermo Scientific Chemicals
Di-n-butyltin dilaurate is used as a catalyst for the production of polyurethanes as well as for the transesterification reactions. It is involved in the vulcanization of silicones and a stabilizer in polyvinyl chloride (PVC).
Di-n-butyltin dilaurate, 95% Thermo Scientific Chemicals
Di-n-butyltin dilaurate is used as a catalyst for the production of polyurethanes as well as for the transesterification reactions. It is involved in the vulcanization of silicones and a stabilizer in polyvinyl chloride (PVC).
Di-n-butyltin dilaurate, 95% Thermo Scientific Chemicals
Di-n-butyltin dilaurate is used as a catalyst for the production of polyurethanes as well as for the transesterification reactions. It is involved in the vulcanization of silicones and a stabilizer in polyvinyl chloride (PVC).
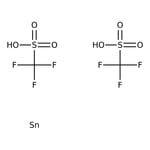
Tin(II) trifluoromethanesulfonate, 97% Thermo Scientific Chemicals
Tin(II) trifluoromethanesulfonate is a mild Lewis acid used for stereoselective aldol and Michael reactions; [2,3]-Wittig and Ireland-Claisen rearrangements, Horner-Wadsworth-Emmons reactions, asymmetric [3 + 2] cycloaddition, ring opening reactions and Friedel-Crafts reactions.
Tin(II) trifluoromethanesulfonate, 97% Thermo Scientific Chemicals
Tin(II) trifluoromethanesulfonate is a mild Lewis acid used for stereoselective aldol and Michael reactions; [2,3]-Wittig and Ireland-Claisen rearrangements, Horner-Wadsworth-Emmons reactions, asymmetric [3 + 2] cycloaddition, ring opening reactions and Friedel-Crafts reactions.