Search Thermo Fisher Scientific
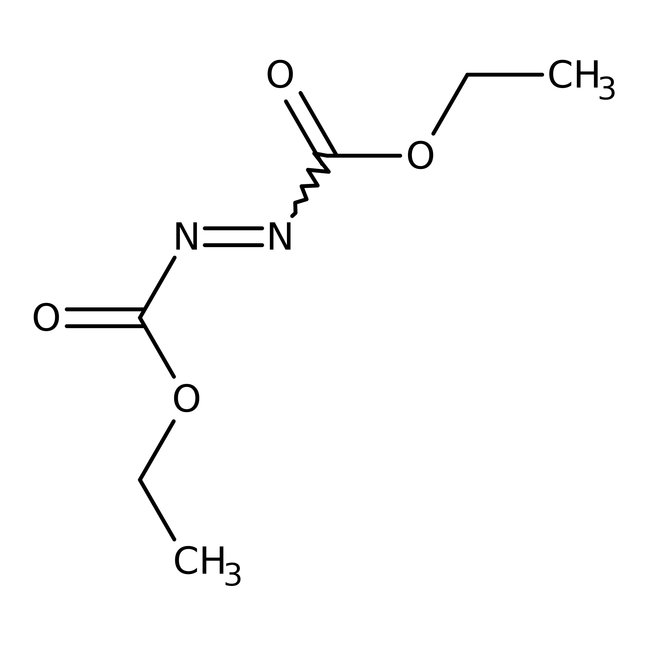
Diethyl azodicarboxylate, 97%, Thermo Scientific™
Catalog number ALFL19348.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or MaterialDiethyl azodicarboxylate
CAS1972-28-7
Health Hazard 1Warning
Health Hazard 2GHS H Statement
H302-H312-H315-H319-H335-H227
Harmful if swallowed.
Harmful in contact with skin.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Combustible liquid.
H302-H312-H315-H319-H335-H227
Harmful if swallowed.
Harmful in contact with skin.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Combustible liquid.
Health Hazard 3GHS P Statement
P210-P261-P280-P305+P351+P338-P405-P501a
Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
Avoid breathing dust/fume/gas/mist/vapors/spray.
Wear protective gloves/protective clothing/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
Store locked up.
Dispose of contents/container in accordance with local/regional/national/international regulations.
P210-P261-P280-P305+P351+P338-P405-P501a
Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
Avoid breathing dust/fume/gas/mist/vapors/spray.
Wear protective gloves/protective clothing/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.
Store locked up.
Dispose of contents/container in accordance with local/regional/national/international regulations.
View more
This Thermo Scientific brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific.
Applications
Diethyl azodicarboxylate acts as a dienophile used in Diels-Alder reactions. It is used as a reagent in alfa-thiocyanation of enolizable ketones with ammonium thiocyanate and annulation of N-protected imines. It acts as a reactant for the preparation of immunostimulants alfa-Galactosylceramides, bisubstrate inhibitors with molecular recognition at the active site of catechol-O-methyltransferase and aza-beta-lactams via NHC-catalyzed [2 + 2] cycloaddition with ketenes. It finds application in Mitsunobu reaction and used in the synthesis of pharmaceuticals like zidovudine and procarbazine. The presence of azo group in it is a Michael acceptor and used for the conversion of beta-ketoesters to the corresponding hydrazine derivatives in presence of copper(II) catalyst. Further, it is an efficient dehydrogenating agent, which is involved in the preparation of aldehydes, disulfides from alcohols and thiols respectively.
Solubility
Miscible with dichloromethane, diethyl ether and toluene.
Notes
Store in a cool place. Incompatible with strong acids, strong bases, strong oxidizing agents and strong reducing agents.
Diethyl azodicarboxylate acts as a dienophile used in Diels-Alder reactions. It is used as a reagent in alfa-thiocyanation of enolizable ketones with ammonium thiocyanate and annulation of N-protected imines. It acts as a reactant for the preparation of immunostimulants alfa-Galactosylceramides, bisubstrate inhibitors with molecular recognition at the active site of catechol-O-methyltransferase and aza-beta-lactams via NHC-catalyzed [2 + 2] cycloaddition with ketenes. It finds application in Mitsunobu reaction and used in the synthesis of pharmaceuticals like zidovudine and procarbazine. The presence of azo group in it is a Michael acceptor and used for the conversion of beta-ketoesters to the corresponding hydrazine derivatives in presence of copper(II) catalyst. Further, it is an efficient dehydrogenating agent, which is involved in the preparation of aldehydes, disulfides from alcohols and thiols respectively.
Solubility
Miscible with dichloromethane, diethyl ether and toluene.
Notes
Store in a cool place. Incompatible with strong acids, strong bases, strong oxidizing agents and strong reducing agents.
General References:
- Explosion risk by heating undiluted material.
- Dehydrogenating agent and aza-dienophile. Oxidizes alcohols to aldehydes, thiols to disulfides and hydrazo to azo groups: J. Am. Chem. Soc., 88, 2328 (1966). Arylhydroxylamines are converted in high yield to nitroso compounds: Chem. Commun., 199 (1967).
- Undergoes pericyclic reactions with alkenes and dienes via both ene and Diels-Alder processes. For further details and references, see: Encyclopedia of Reagents for Organic Synthesis, L. A. Paquette, Ed., Wiley, Chichester (1995), vol. 3, p. 1790.
- Secondary and tertiary amines have been dealkylated by reaction with DEAD under mild conditions, followed by acid hydrolysis: J. Org. Chem., 38, 1652 (1973).
- In combination with Triphenyl phosphine, L02502 , is widely used in the Mitsunobu reaction for activation of primary and secondary alcohols by formation of the alkoxyphosphonium salts, which behave as versatile alkylating agents under mild SN2 conditions: reactions normally proceed with inversion of configuration. For reviews, see: Synthesis, 1 (1981); Org. React., 42, 335 (1992); Org. Prep. Proced. Int., 28, 127 (1996); Synlett, 1221 (2003); Eur. J. Org. Chem., 2763 (2004):
- For study of the mechanism, see: J. Org. Chem., 67, 1751 (2002).