Search Thermo Fisher Scientific
Thermo Scientific Chemicals
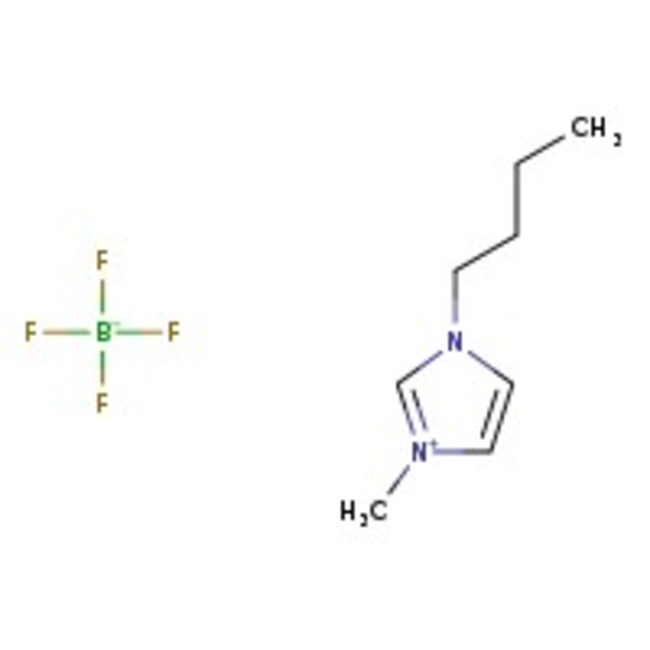
1-n-Butyl-3-methylimidazolium tetrafluoroborate, 98+%, Thermo Scientific Chemicals
CAS: 174501-65-6 | C8H15BF4N2 | 226.03 g/mol
Catalog number ALFL19087.09
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
10 g
Chemical Identifiers
CAS102-79-4
IUPAC Name2-[butyl(2-hydroxyethyl)amino]ethan-1-ol
Molecular FormulaC8H19NO2
InChI KeyGVNHOISKXMSMPX-UHFFFAOYSA-N
SMILESCCCCN(CCO)CCO
View more
Specifications Specification Sheet
Specification Sheet
Refractive index1.4610 to 1.4640 (20°C, 589 nm)
Water=<0.5 %
Appearance (Form)Clear liquid
Infrared spectrumConforms
GC>=97.5 %
View more
1-n-Butyl-3-methylimidazolium tetrafluoroborate is an ionic liquid used for various reactions such as hydrogenations, asymmetric hydrogenations proceed in higher enantioselectivity than in homogeneous phase. It is also used in Suzuki cross-coupling.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-n-Butyl-3-methylimidazolium tetrafluoroborate is an ionic liquid used for various reactions such as hydrogenations, asymmetric hydrogenations proceed in higher enantioselectivity than in homogeneous phase. It is also used in Suzuki cross-coupling.
Solubility
Miscible with acetone, acetonitrile, ethyl acetate, isopropyl alcohol and methylene chloride. Immiscible with hexane, toluene and water.
Notes
Moisture sensitive. Incompatible with strong oxidizing agents.
1-n-Butyl-3-methylimidazolium tetrafluoroborate is an ionic liquid used for various reactions such as hydrogenations, asymmetric hydrogenations proceed in higher enantioselectivity than in homogeneous phase. It is also used in Suzuki cross-coupling.
Solubility
Miscible with acetone, acetonitrile, ethyl acetate, isopropyl alcohol and methylene chloride. Immiscible with hexane, toluene and water.
Notes
Moisture sensitive. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Ionic liquid.
- Effective medium for fluorination of alkyl halides with KF: J. Am. Chem. Soc., 124, 10278 (2002).
- The Suzuki coupling of arylboronic acids with aryl bromides gave improved yields with reduced catalyst concentrations (Tetrakis(triphenyl phosphine) palladium(0) , 10548) with this reaction medium: Chem. Commun., 1249 (2000). Acceleration of Suzuki coupling of polymer-supported 4-iodophenol with arylboronic acids has also been observed: Org. Lett., 4, 3071 (2002).
- Akçay, A.; Babucci, M.; Balci, V.; Uzun, A. A model to predict maximum tolerable temperatures of metal-oxide-supported 1-n-butyl-3-methylimidazolium based ionic liquids. Chem. Eng. Sci. 2015, 123, 588-595.
- Shahid, R.; Muhammad, N.; Gonfa, G.; Toprak, M. S.; Muhammed, M. Synthesis, COSMO-RS analysis and optical properties of surface modified ZnS quantum dots using ionic liquids. J. Phys. Chem. Solids 2015, 85, 34-38.