Search Thermo Fisher Scientific
Thermo Scientific Chemicals
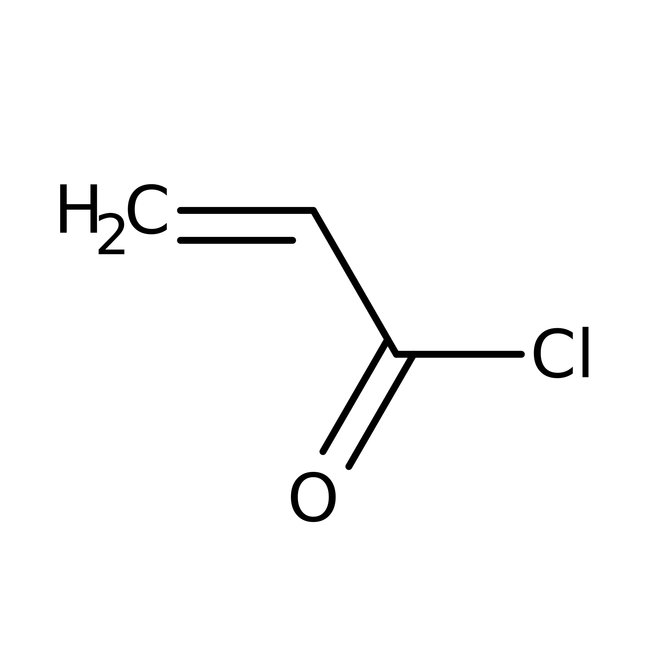
Acryloyl chloride, 96%, stab. with 400ppm phenothiazine, Thermo Scientific Chemicals
CAS: 814-68-6 | C3H3ClO | 90.51 g/mol
Catalog number ALFL10363.09
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
10 g
Chemical Identifiers
CAS2338-18-3
IUPAC Name2,3-dihydro-1H-inden-2-amine hydrochloridyl
Molecular FormulaC9H11ClN
InChI KeyDLYRFJDJKGGVKT-UHFFFAOYSA-N
SMILES[Cl].NC1CC2=CC=CC=C2C1
View more
Specifications Specification Sheet
Specification Sheet
FormCrystals or powder or crystalline powder
Assay (Titration ex Chloride)≥97.5 to ≤102.5%
Appearance (Color)White to cream to yellow
Acryloyl chloride is used in the production of plastics. It plays an important role in the preparation of acrylate monomers and polymers. It also acts as a substrate for cross-metathesis. Further, it is utilized in organic synthesis for the introduction of acrylic groups into other compounds.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Acryloyl chloride is used in the production of plastics. It plays an important role in the preparation of acrylate monomers and polymers. It also acts as a substrate for cross-metathesis. Further, it is utilized in organic synthesis for the introduction of acrylic groups into other compounds.
Solubility
Miscible with water.
Notes
Moisture and light sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with alcohols, oxidizing agents, strong bases and polymerizing initiators.
Acryloyl chloride is used in the production of plastics. It plays an important role in the preparation of acrylate monomers and polymers. It also acts as a substrate for cross-metathesis. Further, it is utilized in organic synthesis for the introduction of acrylic groups into other compounds.
Solubility
Miscible with water.
Notes
Moisture and light sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with alcohols, oxidizing agents, strong bases and polymerizing initiators.
RUO – Research Use Only
General References:
- Warneke, J.; Plaumann, M.; Wang, Z.; Böhler, E.; Kemken, D.; Kelm, S.; Leibfritz, D.; Azov, V. A. New insights into the old reaction between acryloyl chlorides and pyridine. Tetrahedron Lett. 2015, 56 (9), 1124-1127.
- Lee, P. W.; Scrape, P. G.; Butler, L. G.; Lee, Y. P. Two HCl-Elimination Channels and Two CO-Formation Channels Detected with Time-Resolved Infrared Emission upon Photolysis of Acryloyl Chloride [CH2CHC(O)Cl] at 193 nm. J. Phys. Chem. A 2015, 119 (28), 7293-7304.