Search Thermo Fisher Scientific
Thermo Scientific Chemicals
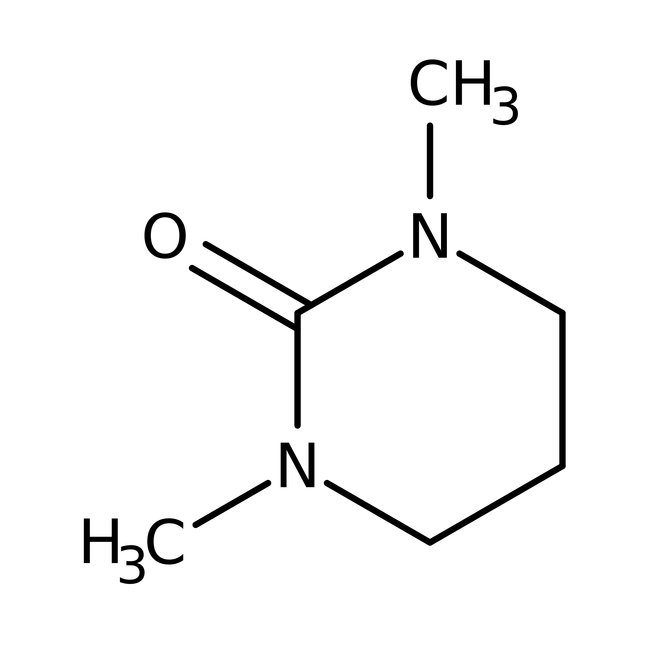
1,3-Dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone, 98%, Thermo Scientific Chemicals
CAS: 7226-23-5 | C6H12N2O | 128.175 g/mol
Catalog number ALFL09968.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Chemical Identifiers
CAS16948-16-6
IUPAC Name(2S)-2-{[(tert-butoxy)carbonyl](methyl)amino}propanoic acid
Molecular FormulaC9H17NO4
InChI KeyVLHQXRIIQSTJCQ-LURJTMIESA-N
SMILESC[C@H](N(C)C(=O)OC(C)(C)C)C(O)=O
View more
Specifications Specification Sheet
Specification Sheet
FormPowder
Melting Point (clear melt)88.0-94.0?C
Appearance (Color)White
Assay (Aqueous acid-base Titration)≥97.5 to ≤102.5%
Optical Rotation-30.4 ± 0.3? (C=1 in ethanol)
1,3-Dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone is a versatile solvent used in the N-alkylation of chiral and O-alkylation of aldoses. It is involved in the preparation of poly(aryl ethers). It is a cyclic urea and used as a polar aprotic organic solvent. Further, it reacts with trifluoro-methanesulfonic acid anhydride to prepare 2,2'-oxy-bis(1,3-dimethyl-tetrahydropyrimidinium) bis(trifluoromethanesulfonate).
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,3-Dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone is a versatile solvent used in the N-alkylation of chiral and O-alkylation of aldoses. It is involved in the preparation of poly(aryl ethers). It is a cyclic urea and used as a polar aprotic organic solvent. Further, it reacts with trifluoro-methanesulfonic acid anhydride to prepare 2,2′-oxy-bis(1,3-dimethyl-tetrahydropyrimidinium) bis(trifluoromethanesulfonate).
Solubility
Miscible with water.
Notes
Hygroscopic. Moisture sensitive. Incompatible with strong oxidizing agents.
1,3-Dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone is a versatile solvent used in the N-alkylation of chiral and O-alkylation of aldoses. It is involved in the preparation of poly(aryl ethers). It is a cyclic urea and used as a polar aprotic organic solvent. Further, it reacts with trifluoro-methanesulfonic acid anhydride to prepare 2,2′-oxy-bis(1,3-dimethyl-tetrahydropyrimidinium) bis(trifluoromethanesulfonate).
Solubility
Miscible with water.
Notes
Hygroscopic. Moisture sensitive. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Dipolar aprotic solvent recommended as a replacement for the carcinogenic hexamethylphosphoric triamide (HMPA) in many reactions: Helv. Chim. Acta, 65, 385 (1982). DMPU is non-mutagenic: Chimia, 39, 148 (1985). It is more useful than the lower homologue 1,3-Dimethyl-2-imidazolidinone, A16001 (mp 8°C) for low-temperature reactions. Can be used as a general replacement for HMPA in organolithium reactions: J. Am. Chem. Soc., 107, 719 (1985).
- Has been found to be comparable to HMPA in promoting reductive coupling reactions with SmI2: J. Am. Chem. Soc., 110, 5064 (1988); J. Chem. Soc., Chem. Commun., 1775 (1992), and also in other reductions with the same reagent: J. Org. Chem., 58, 5008 (1993).
- Nahra, F.; Brill, M.; Gómez-Herrera, A.; Cazin, C. S. J.; Nolan, S. P. Transition metal bifluorides. Coord. Chem. Rev. 2016, 307, 65-80.
- Eagan, J. M.; Hori, M.; Wu, J.; Kanyiva, K. S.; Snyder, S. A. Synthesis and Applications of Hajos-Parrish Ketone Isomers. Angew. Chem. Int. Ed. 2015, 54 (27), 7842-7846.