Search Thermo Fisher Scientific
Thermo Scientific Chemicals
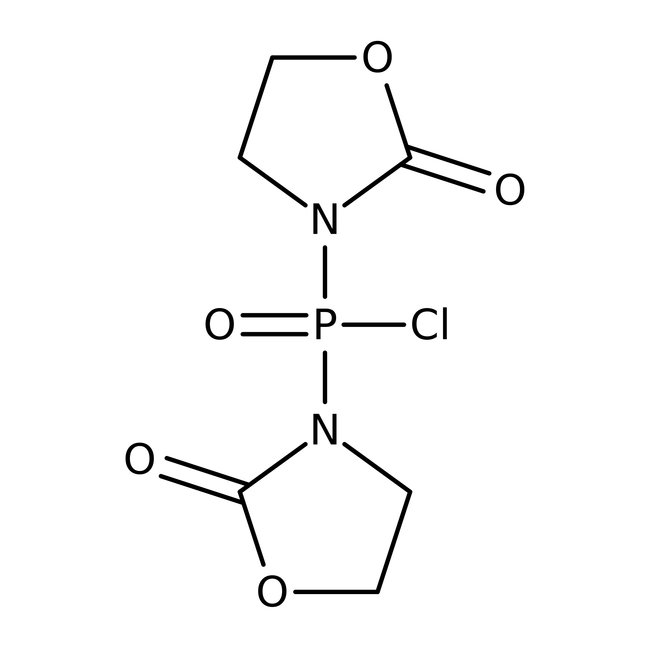
Bis(2-oxo-3-oxazolidinyl)phosphinic chloride, 97%, Thermo Scientific Chemicals
| Catalog Number | Quantity |
|---|---|
| ALFL08775.06 | 5 g |
Catalog number ALFL08775.06
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
5 g
Chemical Identifiers
CAS89-51-0
IUPAC Name2-(carboxymethyl)benzoic acid
Molecular FormulaC9H8O4
InChI KeyZHQLTKAVLJKSKR-UHFFFAOYSA-N
SMILESOC(=O)CC1=CC=CC=C1C(O)=O
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Form)Powder
Melting point178°C to 182°C
Appearance (Color)Off-white to light yellow
Infrared spectrumConforms
HPLC>=97.5 %
View more
Bis(2-oxo-3-oxazolidinyl)phosphinic chloride was used in the preparation of hexadepsipeptide. Also used as reagent is used for activating the carboxylic group, synthesis of amides, esters and peptides.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Bis(2-oxo-3-oxazolidinyl)phosphinic chloride was used in the preparation of hexadepsipeptide. Also used as reagent is used for activating the carboxylic group, synthesis of amides, esters and peptides.
Solubility
Hydrolyzes with water.
Notes
Moisture Sensitive, store away from moisture. Store in cool. Keep container tightly closed in a dry and well-ventilated place. Store away from oxidizing agent.
Bis(2-oxo-3-oxazolidinyl)phosphinic chloride was used in the preparation of hexadepsipeptide. Also used as reagent is used for activating the carboxylic group, synthesis of amides, esters and peptides.
Solubility
Hydrolyzes with water.
Notes
Moisture Sensitive, store away from moisture. Store in cool. Keep container tightly closed in a dry and well-ventilated place. Store away from oxidizing agent.
RUO – Research Use Only
General References:
- T Miyazawa; T Donkai; T Yamada; S Kuwata. Effect of copper(II) chloride on suppression of racemization in peptide synthesis by the mixed anhydride and related methods. International Journal of Peptide and Protein Reseach. 1992, 40 (1), 49-53.
- H T Le; J F Gallard; M Mayer; E Guittet; R Michelot. Use of BOP-Cl in the presence of Boc-amino monothioacids for the thioacylation of imino acid residues. Bioorganic & Medicinal Chemistry. 1996, 4(12), 2201-2209.
- Coupling reagent for which the rates of reaction with various nucleophiles are sufficiently different to permit a genuine one-step coupling of carboxylic acids with amines: Synthesis, 547 (1980). Particularly suitable for coupling N-alkyl amino acids: J. Org. Chem., 51, 3350 (1986). For discussion of strategies for one-step coupling in synthesis of amides, see: Synthesis, 413 (1984), and of the scope and limitations of the reagent in peptide coupling, see: Int. J. Pept. Prot. Res., 29, 574 (1987); J. Org. Chem., 55, 2895 (1990). See also Appendix 6.
- For use in the direct selective 5'-acylation of nucleosides by carboxylic acids, (without 3'-protection), see: Tetrahedron, 44, 229 (1988). For high yield dehydration of carboxylic acids to anhydrides, see: Synthesis, 616 (1981). Also used in macrolide cyclization: J. Am. Chem. Soc., 104, 6818 (1982), and in formation of acyclic esters: Synth. Commun., 14, 515 (1984).
- The mixed anhydride formed with carboxylic acids adds to imines to give ß-lactams: Synthesis, 63 (1982):
- These are also formed in high yield by cyclization of ß-amino acids: Bull. Korean Chem. Soc., 12, 457 (1991).