Search Thermo Fisher Scientific
Thermo Scientific Chemicals
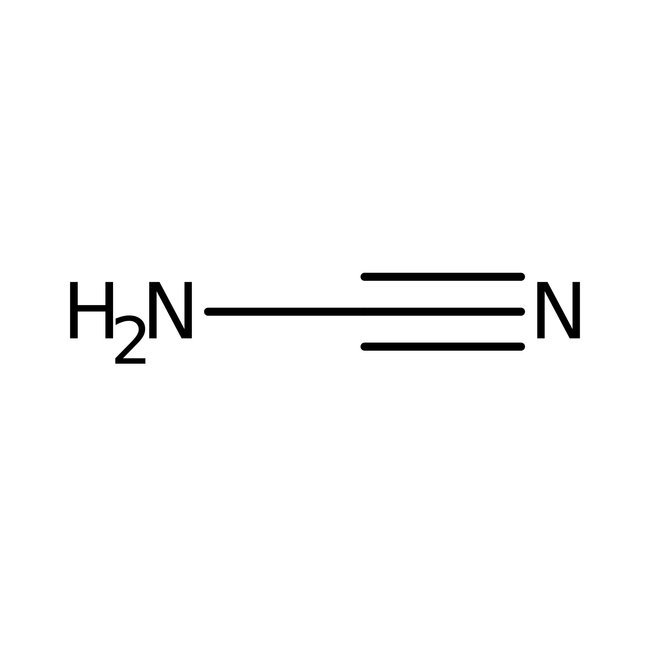
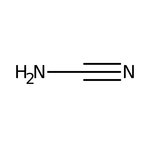
Cyanamide, 98+%, stab., Thermo Scientific Chemicals
CAS: 420-04-2 | CH2N2 | 42.041 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL03744.14 | 25 g |
Catalog number ALFL03744.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or MaterialCyanamide
Name Notestabilized
CAS420-04-2
Health Hazard 1H301+H311-H314-H317-H335-H351-H361fd-H373
Health Hazard 2GHS H Statement
H301-H314-H312-H319-H317
Toxic if swallowed.
Causes severe skin burns and eye damage.
Harmful in contact with skin.
Causes serious eye irritation.
May cause an allergic skin reaction.
H301-H314-H312-H319-H317
Toxic if swallowed.
Causes severe skin burns and eye damage.
Harmful in contact with skin.
Causes serious eye irritation.
May cause an allergic skin reaction.
View more
Cyanamide is used as an intermediate in the production of pharmaceuticals and other organic compounds. It is also used as an alcohol deterrent drug. It is widely used as plant growth regulator, agrochemical and fertilizer.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Cyanamide is used as an intermediate in the production of pharmaceuticals and other organic compounds. It is also used as an alcohol deterrent drug. It is widely used as plant growth regulator, agrochemical and fertilizer.
Solubility
Soluble in water, butanol, methyl ethyl ketone, ethyl acetate, alcohols, phenols, chloroform, amines and ethers. Slightly soluble in carbon disufide. Insoluble in cyclohexane.
Notes
Incompatible with barium peroxide, boric acid, dry hydrogen and hydrogen peroxide.
Cyanamide is used as an intermediate in the production of pharmaceuticals and other organic compounds. It is also used as an alcohol deterrent drug. It is widely used as plant growth regulator, agrochemical and fertilizer.
Solubility
Soluble in water, butanol, methyl ethyl ketone, ethyl acetate, alcohols, phenols, chloroform, amines and ethers. Slightly soluble in carbon disufide. Insoluble in cyclohexane.
Notes
Incompatible with barium peroxide, boric acid, dry hydrogen and hydrogen peroxide.
RUO – Research Use Only
General References:
- Cole, C. A.; Wang, Z.; Snow, T. P.; Bierbaum, V. M. Deprotonated Purine Dissociation: Experiments, Computations, and Astrobiological Implications. J. Phys. Chem. A 2015, 119 (2), 334-343.
- Zhai, P.; Lee, C.; Chang, Y.; Liu, C.; Wei, T.; Feng, S. A Significant Improvement in the Electrocatalytic Stability of N-Doped Graphene Nanosheets Used as a Counter Electrode for [Co(bpy)3]3+/2+ Based Porphyrin-Sensitized Solar Cells. Interfaces 2015, 7 (3), 2116-2123.