Search Thermo Fisher Scientific
Thermo Scientific Chemicals
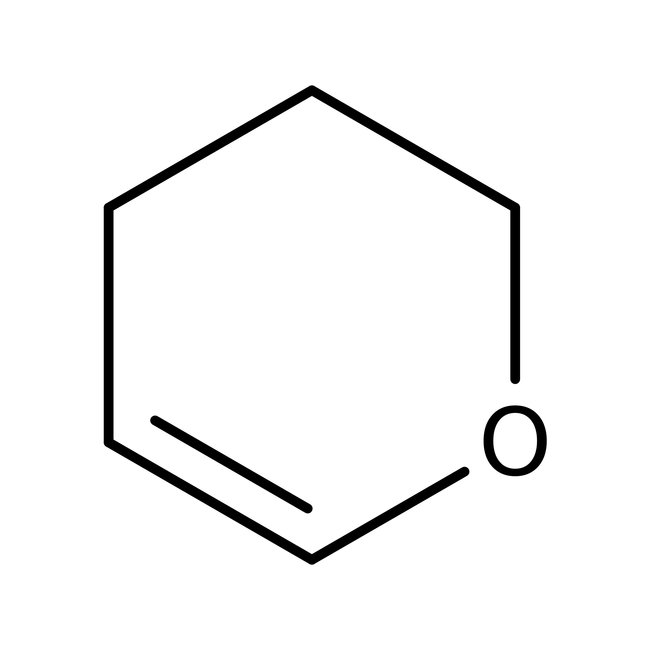
3,4-Dihydro-2H-pyran, 99%, Thermo Scientific Chemicals
CAS: 110-87-2 | C5H8O | 84.118 g/mol
Catalog number ALFL02731.AC
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 mL
Chemical Identifiers
CAS67-66-3
IUPAC Nametrichloromethane
Molecular FormulaCHCl3
InChI KeyHEDRZPFGACZZDS-UHFFFAOYSA-N
SMILESClC(Cl)Cl
View more
Specifications Specification Sheet
Specification Sheet
Color (APHA)10
CommentAcid and chlorine P.T.
Impurity contentFree chlorine (Cl) P.T.
Impurity contentSubstances darkened by sulfuric acid P.T.
CommentAcetone and aldehyde P.T. (limit about 0.005% as (CH₃)₂CO)
View more
Widely used hydroxyl-protecting reagent3,4-Dihydro-2H-pyran is used as a hydroxyl-protecting reagent in organic synthesis. It acts as an intermediate in synthetic chemistry. It is used to protect various reactive functional groups. It is involved in the polymerization reaction either alone or with unsaturated compound and finds application in polymer industries. Further, it is employed in the preparation of bicyclic compounds of epoxide-fused, halo compounds and allenic alcohols.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Widely used hydroxyl-protecting reagent3,4-Dihydro-2H-pyran is used as a hydroxyl-protecting reagent in organic synthesis. It acts as an intermediate in synthetic chemistry. It is used to protect various reactive functional groups. It is involved in the polymerization reaction either alone or with unsaturated compound and finds application in polymer industries. Further, it is employed in the preparation of bicyclic compounds of epoxide-fused, halo compounds and allenic alcohols.
Solubility
Miscible with water and chloroform.
Notes
Incompatible with oxidizing agents, strong acids and alcohols.
Widely used hydroxyl-protecting reagent3,4-Dihydro-2H-pyran is used as a hydroxyl-protecting reagent in organic synthesis. It acts as an intermediate in synthetic chemistry. It is used to protect various reactive functional groups. It is involved in the polymerization reaction either alone or with unsaturated compound and finds application in polymer industries. Further, it is employed in the preparation of bicyclic compounds of epoxide-fused, halo compounds and allenic alcohols.
Solubility
Miscible with water and chloroform.
Notes
Incompatible with oxidizing agents, strong acids and alcohols.
RUO – Research Use Only
General References:
- Protects OH groups as their tetrahydropyranyl, (THP) ethers, stable to bases, Grignard or organolithium reagents, metal hydrides, etc., but readily removed by mild acid. Introduction (acetal formation) generally employs an acid catalyst, often p-TsOH. For example of protection, reaction with Grignard, and deprotection, see: Org. Synth. Coll., 7, 334 (1990). For alternative catalysts for THP ether formations, see: Pyridinium p-toluenesulfonate, A15708, Triphenyl phosphine hydrobromide, L14290, Boron trifluoride diethyl etherate, A15275, Amberlyst™ 15, 89079, Montmorillonite K10, L15160, Acetonyl triphenyl phosphonium bromide, B22434, Indium(III) trifluoromethanesulfonate, 40131. Sulfuric acid/ silica gel: Synth. Commun., 22, 159 (1992), allows the catalyst to be removed by simple filtration. POCl3, described for the protection of diethyl hydroxymethylphosphonate, permits use in the Horner-Wadsworth-Emmons reaction: Org. Synth. Coll., 7, 160 (1990). See also Iodotrimethyl silane, A12902, providing non-acidic conditions; cf also Tetrahydropyran, A13392. For selective monotetrahydropyranylation of symmetrical diols, catalyzed by acidic ion-exchange resin, Dowex™ 50WX2 50-100 (H), L13943, see: J. Org. Chem., 63, 8183 (1998).
- For selective deprotection of THP ethers under non-acidic conditions with LiCl in wet DMSO, see: J. Org. Chem., 61, 6038 (1996).
- The lithio-derivative, formed with tert-BuLi, reacts with, e.g. alkyl iodides or carbonyl compounds: Tetrahedron Lett., 4187 (1977).
- Acetals and ortho esters, in the presence of Lewis acid catalysts, add readily to the vinyl ether system: Chem. Lett., 1101 (1988).