Search Thermo Fisher Scientific
Thermo Scientific Chemicals
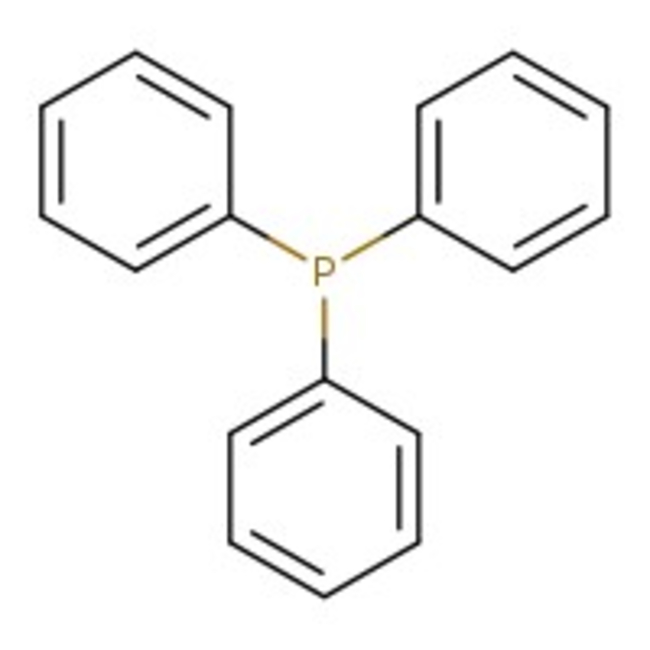
Triphenylphosphine, powder, 99%, Thermo Scientific Chemicals
CAS: 603-35-0 | C18H15P | 262.29 g/mol
Catalog number ALFL02502.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Chemical Identifiers
CAS2610-10-8
IUPAC Namehexasodium (3E)-4-oxo-7-({[(6E)-5-oxo-7-sulfonato-6-(2-{2-sulfonato-4-[2-(4-sulfonatophenyl)diazen-1-yl]phenyl}hydrazin-1-ylidene)-5,6-dihydronaphthalen-2-yl]carbamoyl}amino)-3-(2-{2-sulfonato-4-[2-(4-sulfonatophenyl)diazen-1-yl]phenyl}hydrazin-1-ylidene)-3,4-dihydronaphthalene-2-sulfonate
Molecular FormulaC45H26N10Na6O21S6
InChI KeyLDCKXVAPQFUIOI-ZFLDVXHKSA-H
SMILES[Na+].[Na+].[Na+].[Na+].[Na+].[Na+].[O-]S(=O)(=O)C1=CC=C(C=C1)N=NC1=CC=C(NN=C2/C(=O)C3=CC=C(NC(=O)NC4=CC=C5C(=C4)C=C(C(=NNC4=CC=C(C=C4S([O-])(=O)=O)N=NC4=CC=C(C=C4)S([O-])(=O)=O)C5=O)S([O-])(=O)=O)C=C3C=C2S([O-])(=O)=O)C(=C1)S([O-])(=O)=O
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Dark red. Purple or brown
lambda-max (UV-Vis)524 - 532nm (water)
Solution TestRed solution in water
Formpowder
Triphenylphosphine is used in the synthesis of organic compounds due to its nucleophilicity and its reducing character. It is involved in the synthesis of biaryl compounds, phosphonium salts and other phosphorus compounds. As a reducing agent, it is used to prepare aromatic amines from the corresponding aromatic N-oxides. The anionic phosphine is usually isolated as the trisodium salt, which reacts with rhodium to form a complex that finds use in industrial hydroformylation reactions. It is also used to prepare Wilkinson's catalyst, RhCl(PPh3)3 useful to catalyze the hydrogenation of alkenes and tetrakis(triphenylphosphine)palladium(0) that is widely used to catalyse C-C coupling reactions in organic synthesis.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Triphenylphosphine is used in the synthesis of organic compounds due to its nucleophilicity and its reducing character. It is involved in the synthesis of biaryl compounds, phosphonium salts and other phosphorus compounds. As a reducing agent, it is used to prepare aromatic amines from the corresponding aromatic N-oxides. The anionic phosphine is usually isolated as the trisodium salt, which reacts with rhodium to form a complex that finds use in industrial hydroformylation reactions. It is also used to prepare Wilkinson′s catalyst, RhCl(PPh3)3 useful to catalyze the hydrogenation of alkenes and tetrakis(triphenylphosphine)palladium(0) that is widely used to catalyse C-C coupling reactions in organic synthesis.
Solubility
Soluble in ether, benzene, carbon tetrachloride, glacial acetic acid, acetone, chloroform and alcohol. Insoluble in water.
Notes
Incompatible with oxidizing agents and acids.
Triphenylphosphine is used in the synthesis of organic compounds due to its nucleophilicity and its reducing character. It is involved in the synthesis of biaryl compounds, phosphonium salts and other phosphorus compounds. As a reducing agent, it is used to prepare aromatic amines from the corresponding aromatic N-oxides. The anionic phosphine is usually isolated as the trisodium salt, which reacts with rhodium to form a complex that finds use in industrial hydroformylation reactions. It is also used to prepare Wilkinson′s catalyst, RhCl(PPh3)3 useful to catalyze the hydrogenation of alkenes and tetrakis(triphenylphosphine)palladium(0) that is widely used to catalyse C-C coupling reactions in organic synthesis.
Solubility
Soluble in ether, benzene, carbon tetrachloride, glacial acetic acid, acetone, chloroform and alcohol. Insoluble in water.
Notes
Incompatible with oxidizing agents and acids.
RUO – Research Use Only
General References:
- Finds widespread use as a complexing agent with transition metals, either in the preparation of stable complexes, or as an additive in metal-promoted reactions. See also Tri(o-tolyl) phosphine, A12093 and Tri(2-furyl) phosphine, L13329.
- Azides can be converted selectively to amines, in the presence of ester, epoxide or nitro groups, by reduction to iminophosphoranes (Staudinger reaction), followed by hydrolysis. For reviews, see: Tetrahedron, 37, 437 (1981); 48, 1353 (1992):
- In suitable circumstances, the iminophosphorane may undergo intramolecular aza-Wittig reaction: Tetrahedron, 45, 6375 (1989):
- Reviews: Aza-Wittig reaction in heterocyclic synthesis: Org. Prep. Proced. Int., 24, 209 (1992); Iminophosphoranes as building blocks for the preparation of N-containing heterocycles: Synthesis, 1197 (1994).
- For use in peptide and other coupling reactions, see 2,2'-Dipyridyl disulfide, A11118.
- For use in the Mitsunobu reaction for conversion of alcohols to alkylating agents, see N-Guanyl urea sulfate, A19106, and Diisopropyl azodicarboxyl ate, L10386. See also 1,2-Bis(diphenyl phosphino) ethane, A11419.
- Loghmani, M. H.; Shojaei, A. F. Reduction of cobalt ion improved by lanthanum and zirconium as a triphenylphosphine stabilized nano catalyst for hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2015, 40 (20), 6573-6581.
- Nirmala, M.; Prakash, G.; Viswanathamurthi, P.; Malecki, J. G. An attractive route to transamidation catalysis: Facile synthesis of new o-aryloxide-N-heterocyclic carbene ruthenium (II) complexes containing trans triphenylphosphine donors. J. Mol. Catal. A: Chem. 2015, 403, 15-26.