Search Thermo Fisher Scientific
Thermo Scientific Chemicals
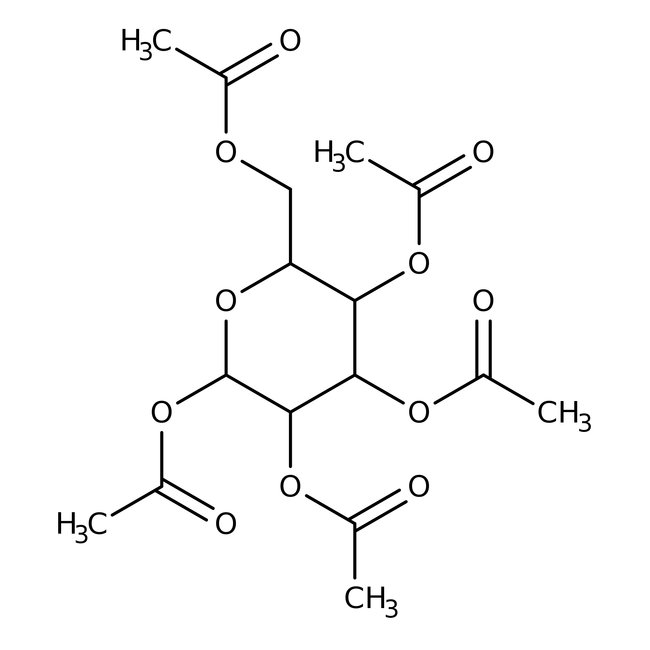
1,2,3,4,6-Penta-O-acetyl-D-mannopyranose, 98%
CAS: 25941-03-1 | C16H22O11 | 390.341 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFJ66973.22 | 100 g |
Catalog number ALFJ66973.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
Chemical Name or Material1,2,3,4,6-Penta-O-acetyl-D-mannopyranose
CAS25941-03-1
Melting Point66°C to 72°C
ColorWhite
Recommended StorageKeep cold
View more
1,2,3,4,6-Penta-O-acetyl-D-mannopyranose is used for the preparation of glycopyranoside phosphates for the study of T-lymphocyte mediated inflammatory diseases.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,2,3,4,6-Penta-O-acetyl-D-mannopyranose is used for the preparation of glycopyranoside phosphates for the study of T-lymphocyte mediated inflammatory diseases.
Solubility
Soluble in dichloromethane, ether, ethyl acetate, methanol, and chloroform.
Notes
Keep container tightly closed in a dry and well-ventilated place. Store in cool place. Store at -20°C.
1,2,3,4,6-Penta-O-acetyl-D-mannopyranose is used for the preparation of glycopyranoside phosphates for the study of T-lymphocyte mediated inflammatory diseases.
Solubility
Soluble in dichloromethane, ether, ethyl acetate, methanol, and chloroform.
Notes
Keep container tightly closed in a dry and well-ventilated place. Store in cool place. Store at -20°C.
RUO – Research Use Only
General References:
- Qingbing Wang.; Jie Fu.; Jianbo Zhang. A facile preparation of peracylated α-aldopyranosyl chlorides with thionyl chloride and tin tetrachloride. Carbohydrate Research. 2008, 343, (17), 2989-2991.
- Sanjoy K Das.; M.Corazon Trono.; René Roy. Transition Metal-Catalyzed Cross-Coupling Reactions toward the Synthesis of α-d-Mannopyranoside Clusters. Methods in Enzymology. 2003, 362, 3-17.