Search Thermo Fisher Scientific
Thermo Scientific Chemicals
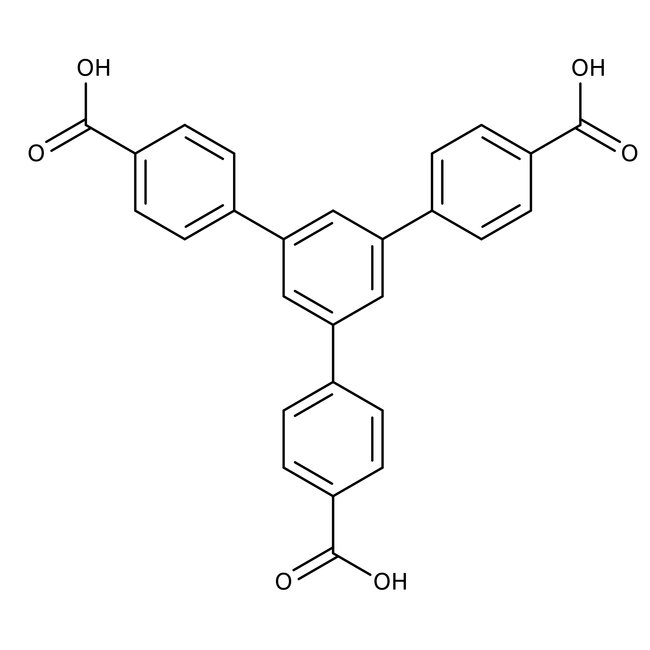
1,3,5-Tri(4-carboxyphenyl)benzene, 97%, Thermo Scientific Chemicals
CAS: 50446-44-1 | C27H18O6 | 438.44 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFH60087.06 | 5 g |
Catalog number ALFH60087.06
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
5 g
Specifications
Chemical Name or Material1,3,5-Tri(4-carboxyphenyl)benzene
CAS50446-44-1
Health Hazard 1H315-H319
Health Hazard 2GHS H Statement
H315-H319
Causes skin irritation.
Causes serious eye irritation.
H315-H319
Causes skin irritation.
Causes serious eye irritation.
Health Hazard 3P264b-P280-P302+P352-P305+P351+P338-P332+P313-P362
View more
1,3,5-Tri(4-carboxyphenyl)benzene is used as a building block for metal organic frameworks, which is a 3D-microporous material and finds applications in gas adsorption and separation technologies.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,3,5-Tri(4-carboxyphenyl)benzene is used as a building block for metal organic frameworks, which is a 3D-microporous material and finds applications in gas adsorption and separation technologies.
Solubility
Soluble in tetrahydrofuran.
Notes
Incompatible with strong oxidizing agents, metals, amines, strong acids, acid chlorides, alcohols, peroxides, permanganates, strong reducing agents, soluble carbonates, phosphates, phosphorus halides and hydroxides.
1,3,5-Tri(4-carboxyphenyl)benzene is used as a building block for metal organic frameworks, which is a 3D-microporous material and finds applications in gas adsorption and separation technologies.
Solubility
Soluble in tetrahydrofuran.
Notes
Incompatible with strong oxidizing agents, metals, amines, strong acids, acid chlorides, alcohols, peroxides, permanganates, strong reducing agents, soluble carbonates, phosphates, phosphorus halides and hydroxides.
RUO – Research Use Only
General References:
- Li, D. X.; Ren, Z. G.; Young, D. J.; Lang, J. P. Synthesis of Two Coordination Polymer Photocatalysts and Significant Enhancement of Their Catalytic Photodegradation Activity by Doping with Co2+ Ions. Eur. J. Inorg. Chem. 2015, 2015 (11), 1981-1988.
- Clough, A.; Zheng, S. T.; Zhao, X.; Lin, Q.; Feng, P.; Bu, X. New Lithium Ion Clusters for Construction of Porous MOFs. Cryst. Growth Des. 2014, 14 (3), 897-900.