Search Thermo Fisher Scientific
Thermo Scientific Chemicals
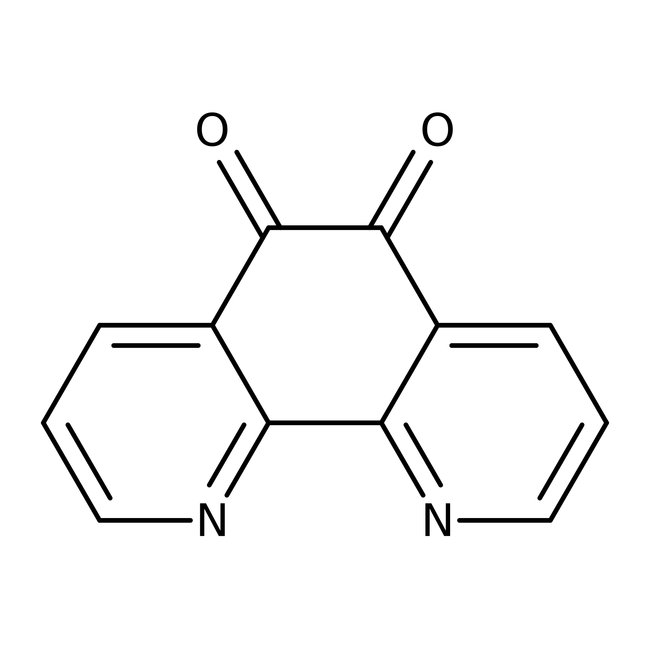
1,10-Phenanthroline-5,6-dione, 98%, Thermo Scientific Chemicals
CAS: 27318-90-7 | C12H6N2O2 | 210.19 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFH55747.03 | 1 g |
Catalog number ALFH55747.03
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
1 g
Chemical Identifiers
CAS36603-49-3
IUPAC Name2-(4-bromophenoxy)oxane
Molecular FormulaC11H13BrO2
InChI KeyMXDQGXMBJCGRCB-UHFFFAOYNA-N
SMILESBrC1=CC=C(OC2CCCCO2)C=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White
FormCrystals or powder or crystalline powder
Assay (GC)≥97.5%
Melting Point (clear melt)53.0-59.0?C
1,10-Phenanthroline-5,6-dione is a Bifunctional quinone oxidant which, when used in conjunction with Zn2+ catalysts, is used to affect the aerobic oxidation of secondary amines to a variety of value added motifs, including indoles.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1,10-Phenanthroline-5,6-dione is a Bifunctional quinone oxidant which, when used in conjunction with Zn2+ catalysts, is used to affect the aerobic oxidation of secondary amines to a variety of value added motifs, including indoles.
Solubility
Insoluble in water.
Notes
Store in a cool and dark place. Store away from incompatible materials such as oxidizing agents.
1,10-Phenanthroline-5,6-dione is a Bifunctional quinone oxidant which, when used in conjunction with Zn2+ catalysts, is used to affect the aerobic oxidation of secondary amines to a variety of value added motifs, including indoles.
Solubility
Insoluble in water.
Notes
Store in a cool and dark place. Store away from incompatible materials such as oxidizing agents.
RUO – Research Use Only
General References:
- Charles A. Goss.; Hector D. Abruna. Spectral, electrochemical and electrocatalytic properties of 1,10-phenanthroline-5,6-dione complexes of transition metals. Inorg. Chem. 1985, 24 (25), 4263-4267.
- Q. Wu.; M. Maskus.; F. Pariente.; F. Tobalina.; V. M. Fernández.; E. Lorenzo.; H. D. Abruña. Electrocatalytic Oxidation of NADH at Glassy Carbon Electrodes Modified with Transition Metal Complexes Containing 1,10-Phenanthroline-5,6-dione Ligands. Anal. Chem. 1996, 68 (20),3688-3696.