Search Thermo Fisher Scientific
Thermo Scientific Chemicals
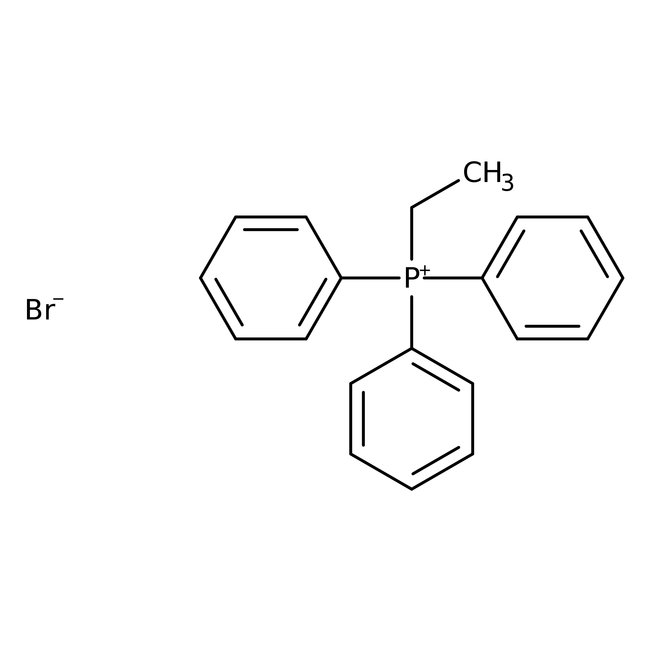
Ethyltriphenylphosphonium bromide, 98+%, Thermo Scientific Chemicals
CAS: 1530-32-1 | C20H20BrP | 371.26 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFB23096.22 | 100 g |
Catalog number ALFB23096.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS2850-65-9
IUPAC Namecopper(2+) diethanolate
Molecular FormulaC4H10CuO2
InChI KeyCRCKGIUJMFFISH-UHFFFAOYSA-N
SMILES[Cu++].CC[O-].CC[O-]
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Blue to blue-green to green
Assay from Supplier's CofA≥97.5%
FormPowder
Ethyltriphenylphosphonium bromide acts as a reactant in the synthesis of D-amino acids from L-cysteine-derived thiazolidines, Leiodolide A through aldol reactions and Horner-Wadsworth-Emmons olefination. It is also used in the preparation of cycloalkanoindolines through diastereoselective intramolecular inimo-ene reactions. Further, it is used as a reagent in solid-state metathesis polycondensation to prepare alkyl-dipropenylthiophene monomers and Mizoroki-Heck cyclization and cascading Tsuji-Trost cyclization reactions.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Ethyltriphenylphosphonium bromide acts as a reactant in the synthesis of D-amino acids from L-cysteine-derived thiazolidines, Leiodolide A through aldol reactions and Horner-Wadsworth-Emmons olefination. It is also used in the preparation of cycloalkanoindolines through diastereoselective intramolecular inimo-ene reactions. Further, it is used as a reagent in solid-state metathesis polycondensation to prepare alkyl-dipropenylthiophene monomers and Mizoroki-Heck cyclization and cascading Tsuji-Trost cyclization reactions.
Solubility
Soluble in methanol. Slightly soluble in water, acetone and isopropanol.
Notes
Hygroscopic. Incompatible with oxidizing agents.
Ethyltriphenylphosphonium bromide acts as a reactant in the synthesis of D-amino acids from L-cysteine-derived thiazolidines, Leiodolide A through aldol reactions and Horner-Wadsworth-Emmons olefination. It is also used in the preparation of cycloalkanoindolines through diastereoselective intramolecular inimo-ene reactions. Further, it is used as a reagent in solid-state metathesis polycondensation to prepare alkyl-dipropenylthiophene monomers and Mizoroki-Heck cyclization and cascading Tsuji-Trost cyclization reactions.
Solubility
Soluble in methanol. Slightly soluble in water, acetone and isopropanol.
Notes
Hygroscopic. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Reaction of the phosphorane (generated using 1 mole of n-BuLi) with an aldehyde at low temperature, followed by a second mole of n-BuLi, gives the -oxido phosphonium ylide which can then be reacted with 1,2-diiodoethane to give (Z)-2-iodo-2-alkenes with high stereoselectivity: J. Chem. Soc., Perkin 1, 1331 (1995). See Appendix 1.
- Bagh, F. S. G.; Shahbaz, K.; Mjalli, F. S.; Hashim, M. A.; AlNashef, I. M. Zinc(II) chloride-based deep eutectic solvents for application as electrolytes: Preparation and characterization. J. Mol. Liq. 2015, 204, 76-83.
- Harada, K.; Imai, A.; Uto, K.; Carter, R. G.; Kubo, M.; Hioki, H.; Fukuyama, Y. Synthesis of jiadifenin using Mizoroki-Heck and Tsuji-Trost reactions. Tetrahedron 2015, 71 (15), 2199-2209.