Search Thermo Fisher Scientific
Thermo Scientific Chemicals
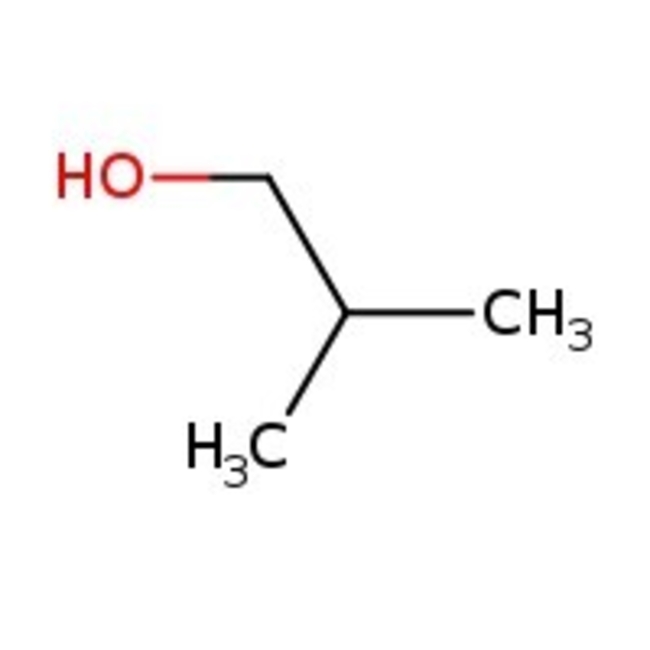
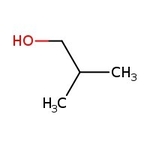
Isobutanol, 99%, Thermo Scientific Chemicals
CAS: 78-83-1 | C4H10O | 74.123 g/mol
Catalog number ALFB23091.AP
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 mL
Chemical Identifiers
CAS7148-74-5
IUPAC Name2-bromopropanoyl chloride
Molecular FormulaC3H4BrClO
InChI KeyOZGMODDEIHYPRY-UHFFFAOYNA-N
SMILESCC(Br)C(Cl)=O
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Form)Liquid
Refractive index1.4770 to 1.4810 (20°C, 589 nm)
Titration Argentometric>=97.5 % (ex Cl)
Infrared spectrumConforms
Appearance (Color)Clear yellow
Isobutanol is a reagent used in organic reactions. It is employed in the synthesis of new fluorinating reagents. It is also used in the lipase-catalyzed production of biodiesel as an energy source. It acts as a catalyst for the liquid-phase esterification of acetic acid by ion-exchange resins. Dehydration of fermented isobutanol is useful in the production of renewable chemicals and fuels.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Isobutanol is a reagent used in organic reactions. It is employed in the synthesis of new fluorinating reagents. It is also used in the lipase-catalyzed production of biodiesel as an energy source. It acts as a catalyst for the liquid-phase esterification of acetic acid by ion-exchange resins. Dehydration of fermented isobutanol is useful in the production of renewable chemicals and fuels.
Solubility
Miscible with alcohol and diethyl ether. Partially miscible with water.
Notes
Avoid all sources of ignition. Keep container tightly closed in a dry and well-ventilated place.
Isobutanol is a reagent used in organic reactions. It is employed in the synthesis of new fluorinating reagents. It is also used in the lipase-catalyzed production of biodiesel as an energy source. It acts as a catalyst for the liquid-phase esterification of acetic acid by ion-exchange resins. Dehydration of fermented isobutanol is useful in the production of renewable chemicals and fuels.
Solubility
Miscible with alcohol and diethyl ether. Partially miscible with water.
Notes
Avoid all sources of ignition. Keep container tightly closed in a dry and well-ventilated place.
RUO – Research Use Only
General References:
- Akita, H.; Nakashima, N.; Hoshino. T. Bacterial production of isobutanol without expensive reagents. Appl. Microbiol. Biotechnol. 2015, 99 (2), 991-999.
- Dhanala, V.; Maity, S. K.; Shee, D. Oxidative steam reforming of isobutanol over Ni/γ-Al2O3 catalysts: A comparison with thermodynamic equilibrium analysis. J. Ind. Eng. Chem. 2015, 27, 153-163.