Search Thermo Fisher Scientific
Thermo Scientific Chemicals
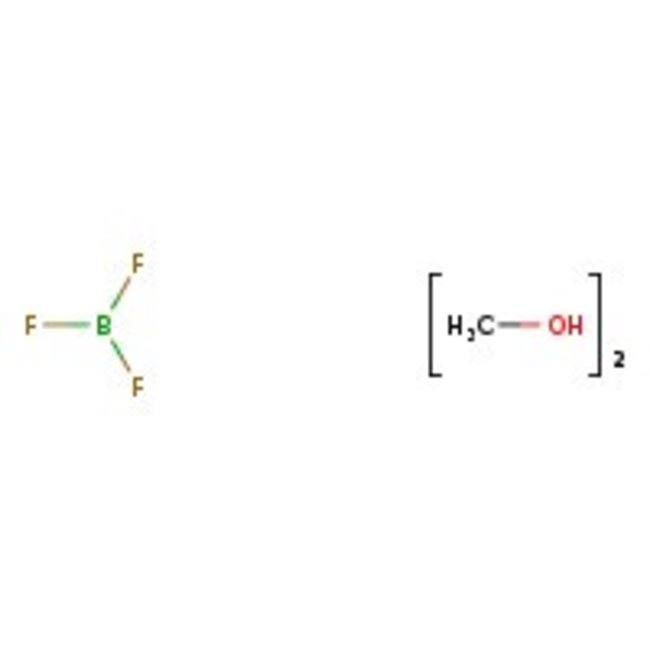
Boron trifluoride-dimethanol complex, 50-52% w/w boron trifluoride, Thermo Scientific Chemicals
CAS: 2802-68-8 | C2H8BF3O2 | 131.89 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFB21357.AK | 250 mL |
Catalog number ALFB21357.AK
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 mL
Specifications
Chemical Name or MaterialBoron trifluoride-dimethanol complex
CAS2802-68-8
Health Hazard 1H227-H302-H311+H331-H314-H335-H370-H372
Health Hazard 2GHS H Statement
H300-H310-H330-H314-H318-H227
Fatal if swallowed.
Fatal in contact with skin.
Fatal if inhaled.
Causes severe skin burns and eye damage.
Causes serious eye damage.
Combustible liquid.
H300-H310-H330-H314-H318-H227
Fatal if swallowed.
Fatal in contact with skin.
Fatal if inhaled.
Causes severe skin burns and eye damage.
Causes serious eye damage.
Combustible liquid.
Health Hazard 3P210-P235-P260-P264b-P270-P271-P280-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P370+P378q-P501c
View more
It finds it application as a reagent for esterification of acids and also for the selective cleavage of trityl ethers.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
It finds it application as a reagent for esterification of acids and also for the selective cleavage of trityl ethers.
Solubility
Hydrolyzes in water.
Notes
^=50% boron fluoride in methanol
It finds it application as a reagent for esterification of acids and also for the selective cleavage of trityl ethers.
Solubility
Hydrolyzes in water.
Notes
^=50% boron fluoride in methanol
RUO – Research Use Only
General References:
- Keith Graham, et.al. Development of a fast robust derivatization method of an extremely polar PET radiopharmaceutical: a critical aspect for starting a clinical trial.Tetrahedron Lett.201354(21), 2583-2586.
- Mizuno, K, et al. Elucidation of formation mechanism of by-products of copper sulfide deposition on insulating paper in oil-immersed transformer.Dielectrics and Electrical Insulation., 201421(3), 1376-1383.
- Reagent for esterification of acids: J. Chem. Soc., 5770 (1965); and also for the selective cleavage of trityl ethers: Carbohydr. Res., 65, 132 (1978).