Search Thermo Fisher Scientific
Thermo Scientific Chemicals
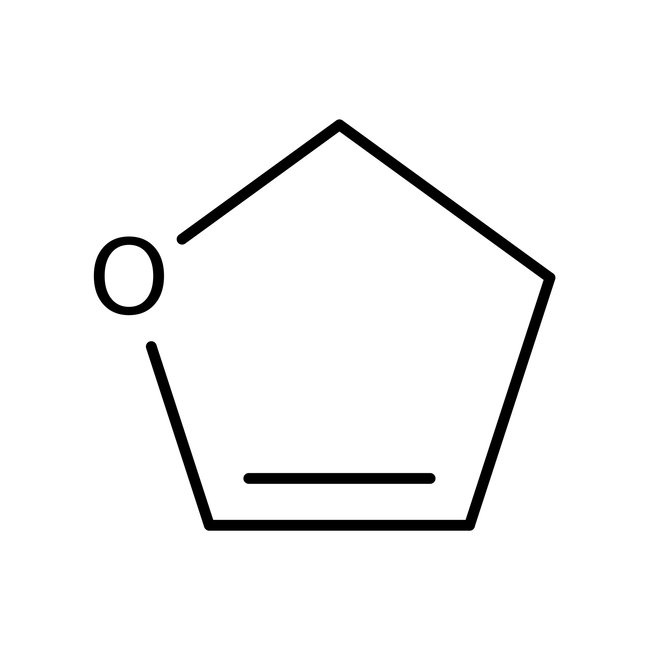
2,3-Dihydrofuran, 98+%, Thermo Scientific Chemicals
CAS: 1191-99-7 | C4H6O | 70.09 g/mol
Catalog number ALFB20575.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS12233-56-6
IUPAC Nametetrabismuth trigermanium dodecahydrate
Molecular FormulaBi4Ge3H24O12
InChI KeyORCSMBGZHYTXOV-UHFFFAOYSA-N
SMILESO.O.O.O.O.O.O.O.O.O.O.O.[Ge].[Ge].[Ge].[Bi].[Bi].[Bi].[Bi]
View more
Specifications Specification Sheet
Specification Sheet
Total Metal Impurities0.0005% max.
2,3-Dihydrofuran is a versatile reagent used in lanthanide-catalyzed Diels-Alder reactions with 2-pyrones and in Rh(II)-stabilized cycloadditions with vinylcarbenoids. It is applied for alkylation by active methylene compounds, catalyzed by trans-Dichlorobis(triphenylphosphine)palladium(II). It is also used in the preparation of biologically active compounds such as antitumor agents.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2,3-Dihydrofuran is a versatile reagent used in lanthanide-catalyzed Diels-Alder reactions with 2-pyrones and in Rh(II)-stabilized cycloadditions with vinylcarbenoids. It is applied for alkylation by active methylene compounds, catalyzed by trans-Dichlorobis(triphenylphosphine)palladium(II). It is also used in the preparation of biologically active compounds such as antitumor agents.
Solubility
Soluble in water at 20°C, 20g/L and chloroform.
Notes
Store away from oxidizing agents and air. Keep the container tightly closed and place it in a cool, dry and well ventilated condition. Protect from heat. Keep it refrigerated.
2,3-Dihydrofuran is a versatile reagent used in lanthanide-catalyzed Diels-Alder reactions with 2-pyrones and in Rh(II)-stabilized cycloadditions with vinylcarbenoids. It is applied for alkylation by active methylene compounds, catalyzed by trans-Dichlorobis(triphenylphosphine)palladium(II). It is also used in the preparation of biologically active compounds such as antitumor agents.
Solubility
Soluble in water at 20°C, 20g/L and chloroform.
Notes
Store away from oxidizing agents and air. Keep the container tightly closed and place it in a cool, dry and well ventilated condition. Protect from heat. Keep it refrigerated.
RUO – Research Use Only
General References:
- Ulrike Kohlweyer, et al. Tetrahydrofuran degradation by a newly isolated culture of Pseudonocardia sp. strain K1.FEMS Microbiol. Lett.2000,186301-306.
- Fumiyuki Ozawa, et al. Catalytic asymmetric arylation of 2,3-dihydrofuran with aryl triflates.J. Am. Chem. Soc.,1991,113(4), 1417-1419.
- For lithiation at the 5-position, see: Tetrahedron Lett., 4187 (1977); Org. Synth. Coll., 9, 530 (1998). For alkylation by active methylene compounds, catalyzed by trans-Dichlorobis(triphenyl phosphine) palladium(II) , 10491, see: J. Org. Chem., 47, 2812 (1982). For catalytic asymmetric arylation, see: J. Am. Chem. Soc., 113, 1417 (1991); Pure Appl. Chem., 64, 421 (1992).
- Forms tetrahydrofuranyl esters (acyl hemiacetals) with carboxylic acids. These react with Grignard reagents to give good yields of ketones with minimal enolization or double addition (tertiary alcohol formation). The utility of other acyl hemiacetals as ketone precursors has also been studied: J. Org. Chem., 61, 6071 (1996):