Search Thermo Fisher Scientific
Thermo Scientific Chemicals
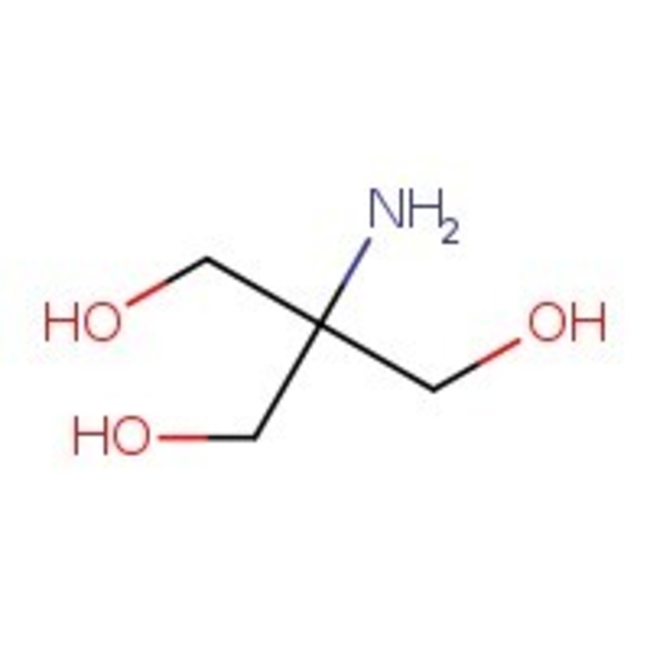
Tris(hydroxymethyl)aminomethane, 99%, Thermo Scientific Chemicals
CAS: 77-86-1 | C4H11NO3 | 121.136 g/mol
Catalog number ALFA18494.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Chemical Identifiers
CAS21324-39-0
IUPAC Namesodium hexafluoro-λ⁵-phosphanuide
Molecular FormulaF6NaP
InChI KeyKMADQUOCJBLXRP-UHFFFAOYSA-N
SMILES[Na+].F[P-](F)(F)(F)(F)F
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to light grey
Assay>=98.50 %
Appearance (Form)Crystalline powder
Tris(hydroxymethyl)aminomethane is used as buffers in biochemistry and molecular biology laboratories. It is used as a primary standard to standardize acid solutions for chemical analysis. It finds application in cell membranes to increase its permeability. As an alternative to sodium bicarbonate, it used in the treatment of metabolic acidosis.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Tris(hydroxymethyl)aminomethane is used as buffers in biochemistry and molecular biology laboratories. It is used as a primary standard to standardize acid solutions for chemical analysis. It finds application in cell membranes to increase its permeability. As an alternative to sodium bicarbonate, it used in the treatment of metabolic acidosis.
Solubility
Soluble in water, ethylene glycol, methanol, ethanol,dimethyl formamide, acetone, ethyl acetate, olive oil, chloroform and alkaline solution.
Notes
Hygroscopic. Incompatible with bases and strong oxidizing agents.
Tris(hydroxymethyl)aminomethane is used as buffers in biochemistry and molecular biology laboratories. It is used as a primary standard to standardize acid solutions for chemical analysis. It finds application in cell membranes to increase its permeability. As an alternative to sodium bicarbonate, it used in the treatment of metabolic acidosis.
Solubility
Soluble in water, ethylene glycol, methanol, ethanol,dimethyl formamide, acetone, ethyl acetate, olive oil, chloroform and alkaline solution.
Notes
Hygroscopic. Incompatible with bases and strong oxidizing agents.
RUO – Research Use Only
General References:
- Widely used biological buffer.
- Filipiak, M. S.; Zloczewska, A.; Grzeskowiak, P.; Lynch, R.; Niedziolka, M. J. Tris (hydroxymethyl) aminomethane photooxidation on titania based photoanodes and its implication for photoelectrochemical biofuel cells. J. Power Sources 2015, 289, 17-21.
- Lee, S. M.; Sim, K. S.; Lo, K. M. Synthesis, characterization and biological studies of diorganotin (IV) complexes with tris [(hydroxymethyl) aminomethane] Schiff bases. Inorg. Chim. Acta 2015, 429, 195-208.