Search Thermo Fisher Scientific
Thermo Scientific Chemicals
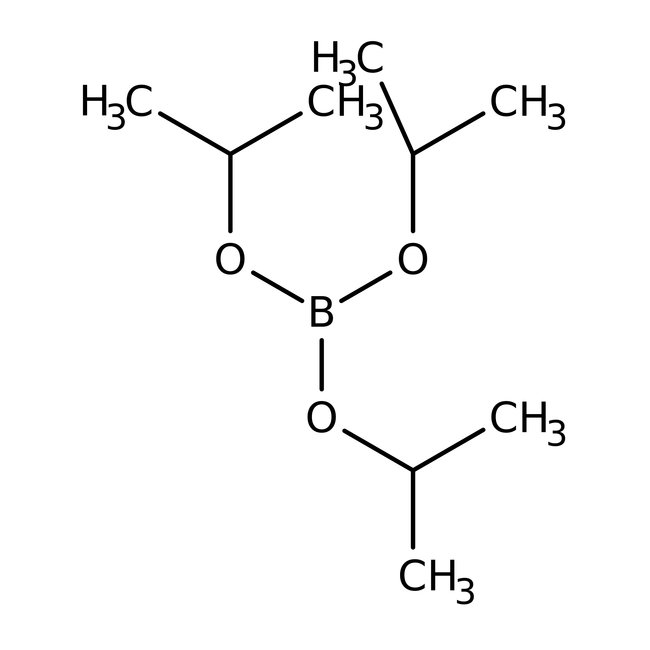
Triisopropyl borate, 98+%, Thermo Scientific Chemicals
CAS: 5419-55-6 | C9H21BO3 | 188.074 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA17592.AP | 500 mL |
Catalog number ALFA17592.AP
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 mL
Specifications
Chemical Name or MaterialTriisopropyl borate
CAS5419-55-6
Health Hazard 1H225
Health Hazard 2GHS H Statement
H225
Highly flammable liquid and vapour.
H225
Highly flammable liquid and vapour.
Health Hazard 3P210-P233-P240-P241-P242-P243-P280-P303+P361+P353-P370+P378q-P501c
View more
Triisopropyl borate is used as reagent in Pd-catalyzed coupling reaction with aryl halides such as Suzuki reaction. It is used as a reagent for the preparation of the boronic acids and esters; as a Lewis acid catalyst and involved in the ortho-borylation of 1-substituted naphthalenes. Furthermore, it plays an important role as a catalyst for the production of resins, waxes, paints and varnishes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Triisopropyl borate is used as reagent in Pd-catalyzed coupling reaction with aryl halides such as Suzuki reaction. It is used as a reagent for the preparation of the boronic acids and esters; as a Lewis acid catalyst and involved in the ortho-borylation of 1-substituted naphthalenes. Furthermore, it plays an important role as a catalyst for the production of resins, waxes, paints and varnishes.
Solubility
Miscible with ether, ethanol, isopropanol and benzene.
Notes
Moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents. Decomposes in water.
Triisopropyl borate is used as reagent in Pd-catalyzed coupling reaction with aryl halides such as Suzuki reaction. It is used as a reagent for the preparation of the boronic acids and esters; as a Lewis acid catalyst and involved in the ortho-borylation of 1-substituted naphthalenes. Furthermore, it plays an important role as a catalyst for the production of resins, waxes, paints and varnishes.
Solubility
Miscible with ether, ethanol, isopropanol and benzene.
Notes
Moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents. Decomposes in water.
RUO – Research Use Only
General References:
- Reagent for preparation of boronates from Grignard or organolithium reagents, often giving higher yields than other trialkyl borates: Organometallics, 2, 1316 (1983); Tetrahedron Lett ., 29, 2631 (1988).
- Garcia-Alonso, D.; Potts, S. E.; van Helvoirt, C. A.; Verheijen, M. A.; Kessels, W. M. Atomic layer deposition of B-doped ZnO using triisopropyl borate as the boron precursor and comparison with Al-doped ZnO. J. Mater. Chem. C 2015, 3 (13), 3095-3107.
- Lyu, S. C.; Han, J. H.; Shin, K. W.; Sok, J. H. Synthesis of boron-doped double-walled carbon nanotubes by the catalytic decomposition of tetrahydrofuran and triisopropyl borate. Carbon 2011, 49 (5), 1532-1541.