Search Thermo Fisher Scientific
Thermo Scientific Chemicals
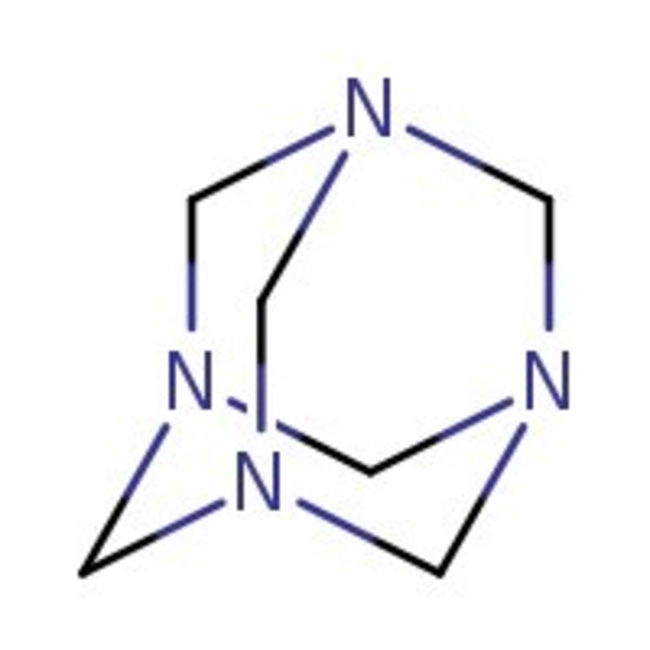
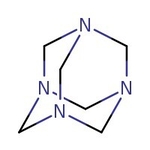
Hexamethylenetetramine, 99+%, Thermo Scientific Chemicals
CAS: 100-97-0 | C6H12N4 | 140.19 g/mol
Catalog number ALFA17213.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Chemical Identifiers
CAS7440-47-3
IUPAC Namechromium
Molecular FormulaCr
InChI KeyVYZAMTAEIAYCRO-UHFFFAOYSA-N
SMILES[Cr]
View more
Hexamethylenetetramine is used as a formylation agent in the synthesis and as a precipitating agent. It is also used in the production of phenolic resins and its moulding compounds, which finds application as binders in fire proof materials, brake and clutch linings. It is involved in the preparation of 5-tert-butyl-2-hydroxy-isophthalaldehyde. It is useful for the treatment of urinary tract infection. Further, it is used in the Duff reaction, Sommelet reaction and in the Delepine reaction.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Hexamethylenetetramine is used as a formylation agent in the synthesis and as a precipitating agent. It is also used in the production of phenolic resins and its moulding compounds, which finds application as binders in fire proof materials, brake and clutch linings. It is involved in the preparation of 5-tert-butyl-2-hydroxy-isophthalaldehyde. It is useful for the treatment of urinary tract infection. Further, it is used in the Duff reaction, Sommelet reaction and in the Delepine reaction.
Solubility
Soluble in water, ethanol, chloroform and acetone.
Notes
Hygroscopic. Incompatible with strong oxidizing agents and strong acids. Avoid conditions like flames, sparks and moisture.
Hexamethylenetetramine is used as a formylation agent in the synthesis and as a precipitating agent. It is also used in the production of phenolic resins and its moulding compounds, which finds application as binders in fire proof materials, brake and clutch linings. It is involved in the preparation of 5-tert-butyl-2-hydroxy-isophthalaldehyde. It is useful for the treatment of urinary tract infection. Further, it is used in the Duff reaction, Sommelet reaction and in the Delepine reaction.
Solubility
Soluble in water, ethanol, chloroform and acetone.
Notes
Hygroscopic. Incompatible with strong oxidizing agents and strong acids. Avoid conditions like flames, sparks and moisture.
RUO – Research Use Only
General References:
- For a review of applications in organic synthesis, see: Synthesis, 161 (1979).
- Benzylic halides form quaternary salts with hexamine which undergo oxidation during hydrolysis to give aldehydes (Sommelet reaction): Org. React., 8, 197 (1954); Org. Synth. Coll., 3, 811 (1955); 4, 690, 918 (1963). Benzylamines can also be converted to benzaldehydes; see e.g.: Org. Synth. Coll., 5, 668 (1973).
- The reaction can also be carried out to give the primary amine on hydrolysis (Delepine reaction). For conversion of allylic halides to amines, see, e.g.: Org. Synth. Coll., 5, 121 (1973); 9, 666 (1998).
- Formylation of arylamines and phenols in the presence of acid to give aldehydes (Duff reaction) generally gives low yields: J. Chem. Soc., 547 (1941); Org. Synth. Coll., 4, 866 (1963). In TFA, formylation of aromatic hydrocarbons occurs in good yield with para-selectivity: J. Org. Chem., 37, 3972 (1972). Careful control of reaction conditions enables 4-substituted phenols in TFA to be converted to either 2-formyl or 2,6-diformyl derivatives: Synthesis, 1029 (1998).
- Can be used as a Mannich reagent, e.g. for the ɑ-methylenation of ketones: Synth. Commun., 26, 1775 (1996). For example with reaction scheme, see Propiophenone, A15140.
- Opasanont, B.; Van, K. T.; Kuba, A. G.; Choudhury, K. R.; Baxter, J. B. Adherent and Conformal Zn(S,O,OH) Thin Films by Rapid Chemical Bath Deposition with Hexamethylenetetramine Additive. ACS Appl. Mater. Interfaces 2015, 7 (21), 11516-11525.
- Durá, G.; Carrión, M. C.; Jalón, F. A.; Manzano, B. R.; Rodríguez, A. M.; Mereiter, K. Robust 2D Coordination Networks from a Two-Step Assembly Process with Predesigned Silver Cyclic Dimers and Hexamethylenetetramine. Cryst. Growth Des. 2015, 15 (7), 3321-3331.