Search Thermo Fisher Scientific
Thermo Scientific Chemicals
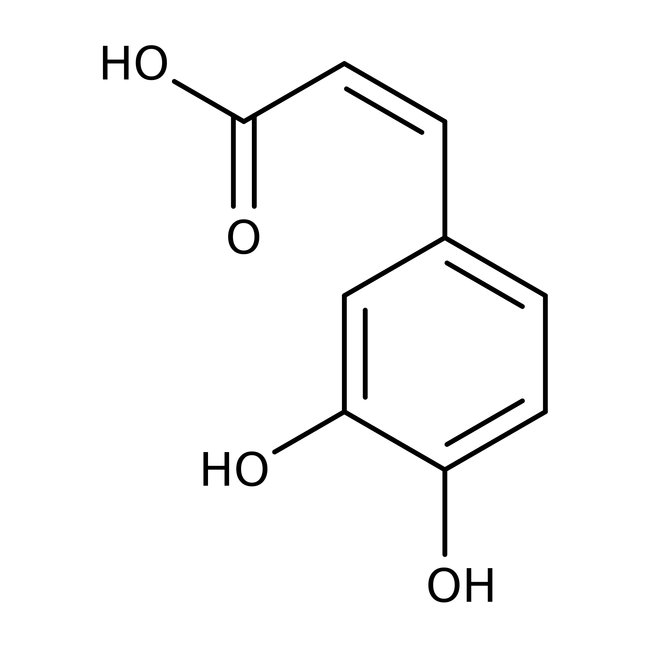
3,4-Dihydroxycinnamic acid, predominantly trans, 98+%, Thermo Scientific Chemicals
CAS: 331-39-5 | C9H8O4 | 180.159 g/mol
Catalog number ALFA15950.18
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
50 g
Chemical Identifiers
CAS108-39-4
IUPAC Name3-methylphenol
Molecular FormulaC7H8O
InChI KeyRLSSMJSEOOYNOY-UHFFFAOYSA-N
SMILESCC1=CC=CC(O)=C1
View more
Specifications Specification Sheet
Specification Sheet
AppearanceMay darken on storage
GC>=98.5 %
Appearance (Color)Clear colorless to light red
Infrared spectrumConforms
Appearance (Form)Viscous liquid
View more
3,4-Dihydroxycinnamic acid, predominantly trans stimulates prostaglandin synthesis at high doses. It has antioxidant, anti-inflammatory, anti-tumor and immunomodulatory properties.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
3,4-Dihydroxycinnamic acid, predominantly trans stimulates prostaglandin synthesis at high doses. It has antioxidant, anti-inflammatory, anti-tumor and immunomodulatory properties.
Solubility
Soluble in DMSO (40 mg/mol), ethanol (25 mg/mL warm), DMF (∼7 mg/mL), PBS, PH 7.2 (∼0.65 mg/mol), hot water, ethyl acetate, Chloroform Hexane, and methanol.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Incompatible with strong bases and strong oxidizing agents.
3,4-Dihydroxycinnamic acid, predominantly trans stimulates prostaglandin synthesis at high doses. It has antioxidant, anti-inflammatory, anti-tumor and immunomodulatory properties.
Solubility
Soluble in DMSO (40 mg/mol), ethanol (25 mg/mL warm), DMF (∼7 mg/mL), PBS, PH 7.2 (∼0.65 mg/mol), hot water, ethyl acetate, Chloroform Hexane, and methanol.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Incompatible with strong bases and strong oxidizing agents.
RUO – Research Use Only
General References:
- Frank Mercer.; Tim Goodman.; Janusz Wojtowicz.; David Duff. Synthesis and characterization of fluorinated aryl ethers prepared from decafluorobiphenyl. Journal of Polymer Science Part A: Polymer Chemistry. 1992, 30 (8), 1767-1770 .
- R Amadelli.; A De Battisti.; D.V Girenko.; S.V Kovalyov.; A.B Velichenko. Electrochemical oxidation of trans-3,4-dihydroxycinnamic acid at PbO2 electrodes: direct electrolysis and ozone mediated reactions compared. Electrochimica Acta. 2000, 46 (2-3), 341-347.