Search Thermo Fisher Scientific
Thermo Scientific Chemicals
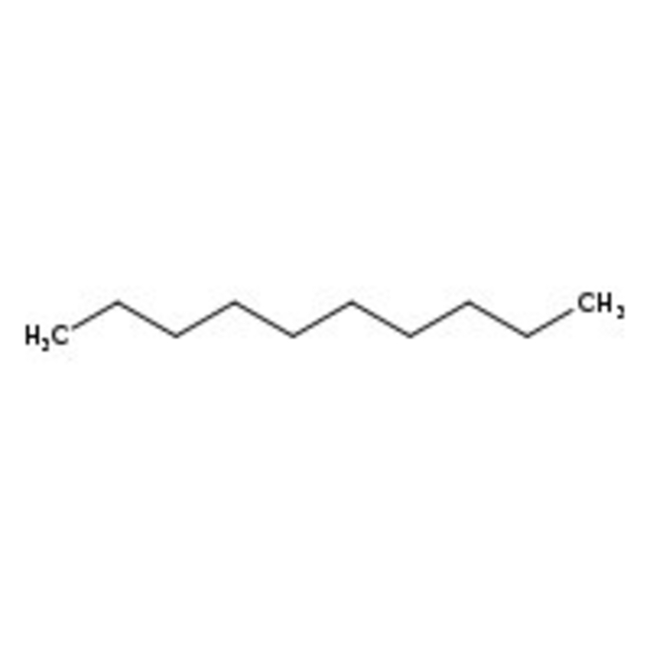
n-Decane, 99%, Thermo Scientific Chemicals
CAS: 124-18-5 | C10H22 | 142.286 g/mol
Catalog number ALFA14732.AP
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 mL
Chemical Identifiers
CAS16961-25-4
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Yellow to orange
Appearance (Form)Powder and/or chunks
AppearanceMay have a brown hue
Insoluble matter=<0.1 % (10 % in ether)
Alkalies+oth.metals (SO4)=<0.2 %
View more
n-Decane is used as a solvent as well as an internal standard in the GC analysis of oxalic, malonic and succinic acids. It is used in paint manufacture as a hydrocarbon solvent and for wood stains and varnishes as a varnish solvent. It is an active component of automotive fuels viz. gasoline (petrol).
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
n-Decane is used as a solvent as well as an internal standard in the GC analysis of oxalic, malonic and succinic acids. It is used in paint manufacture as a hydrocarbon solvent and for wood stains and varnishes as a varnish solvent. It is an active component of automotive fuels viz. gasoline (petrol).
Solubility
Soluble in alcohol and ethers. Miscible with ethanol and ether. Slightly miscible with carbon tetrachloride. Immiscible with water.
Notes
Incompatible with strong oxidizing agents.
n-Decane is used as a solvent as well as an internal standard in the GC analysis of oxalic, malonic and succinic acids. It is used in paint manufacture as a hydrocarbon solvent and for wood stains and varnishes as a varnish solvent. It is an active component of automotive fuels viz. gasoline (petrol).
Solubility
Soluble in alcohol and ethers. Miscible with ethanol and ether. Slightly miscible with carbon tetrachloride. Immiscible with water.
Notes
Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Ji, N.; Wang, X.; Weidenthaler, C.; Spliethoff, B.; Rinaldi, R. Iron(II) Disulfides as Precursors of Highly Selective Catalysts for Hydrodeoxygenation of Dibenzyl Ether into Toluene. Chem. Cat. Chem. 2015, 7 (6), 960-966.
- Khoshsima, A.; Dehghani, M. R.; Touraud, D.; Kunz, W. An investigation of the fish diagrams of water or brine/decane or dodecane/propylene glycol ether (C3P1 or C3P2) systems. J. Mol. Liq. 2015, 206, 170-175.