Search Thermo Fisher Scientific
Thermo Scientific Chemicals
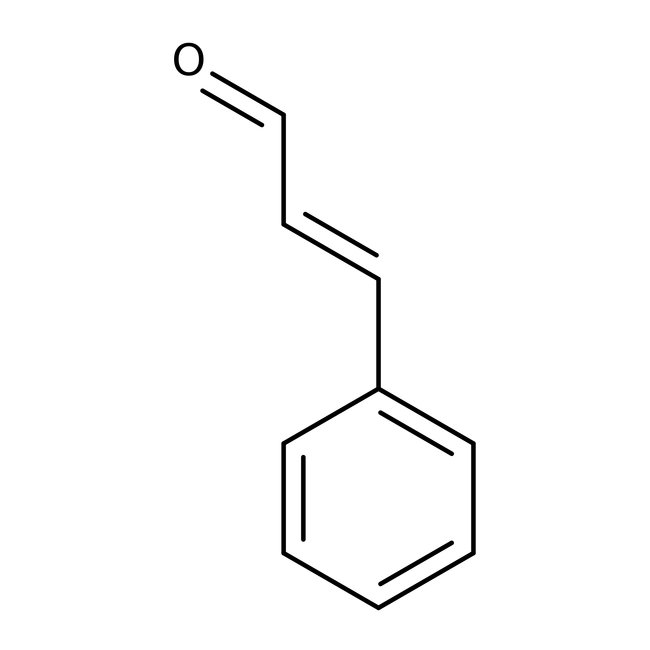
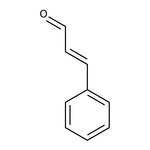
trans-Cinnamaldehyde, 98+%, Thermo Scientific Chemicals
CAS: 14371-10-9 | C9H8O | 132.16 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA14689.22 | 100 g |
Catalog number ALFA14689.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS40663-68-1
IUPAC Name4-(prop-2-en-1-yloxy)benzaldehyde
Molecular FormulaC10H10O2
InChI KeyTYNJQOJWNMZQFZ-UHFFFAOYSA-N
SMILESC=CCOC1=CC=C(C=O)C=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless to pale yellow
Assay (GC)≥96.0%
Refractive Index1.5660-1.5710 @ 20?C
FormLiquid
Free acid (titration)≤1.5%
trans-Cinnamaldehyde is used in the flavor and perfume industry. It is also used in medicine. It reacts with glutathione to get an adduct 1'-(glutathion-S-yl)-dihydrocinnamaldehyde. It is used to prepare cinnamylidene-bisacetamide by reacting with acetamide. Further, it inhibits xanthine oxidase.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
trans-Cinnamaldehyde is used in the flavor and perfume industry. It is also used in medicine. It reacts with glutathione to get an adduct 1′-(glutathion-S-yl)-dihydrocinnamaldehyde. It is used to prepare cinnamylidene-bisacetamide by reacting with acetamide. Further, it inhibits xanthine oxidase.
Solubility
Miscible with water.
Notes
Store in a cool place. Incompatible with strong oxidizing agents and strong bases. Air sensitive.
trans-Cinnamaldehyde is used in the flavor and perfume industry. It is also used in medicine. It reacts with glutathione to get an adduct 1′-(glutathion-S-yl)-dihydrocinnamaldehyde. It is used to prepare cinnamylidene-bisacetamide by reacting with acetamide. Further, it inhibits xanthine oxidase.
Solubility
Miscible with water.
Notes
Store in a cool place. Incompatible with strong oxidizing agents and strong bases. Air sensitive.
RUO – Research Use Only
General References:
- For details of the 1,2-addition of a functionalized Zn-Cu organometallic RCu(CN)ZnI to give an allylic alcohol, see; Org. Synth. Coll., 9, 502 (1998).
- For reaction with 1,2-Ethanedithiol, L12865 to give the dithiolane, followed by coupling with trimethylsilylmethyl magnesium chloride, catalyzed by Dichlorobis(triphenyl phosphine) nickel(II) , 13930, as an example of Ni catalyzed coupling of dithioacetals with Grignards, see: J. Org. Chem., 53, 5582 (1988); Org. Synth. Coll., 9, 727 (1998):
- Zinn, S.; Betz, T.; Medcraft, C.; Schnell, M. Structure determination of trans-cinnamaldehyde by broadband microwave spectroscopy. Phys. Chem. Chem. Phys. 2015, 17, 16080-16085.
- Loquercio, A.; Castell-Perez, E.; Gomes, C.; Moreira, R. G. Preparation of Chitosan-Alginate Nanoparticles for Trans-cinnamaldehyde Entrapment. J. Food Sci. 2015, 80 (10), N2305-N2315.