Search Thermo Fisher Scientific
Thermo Scientific Chemicals
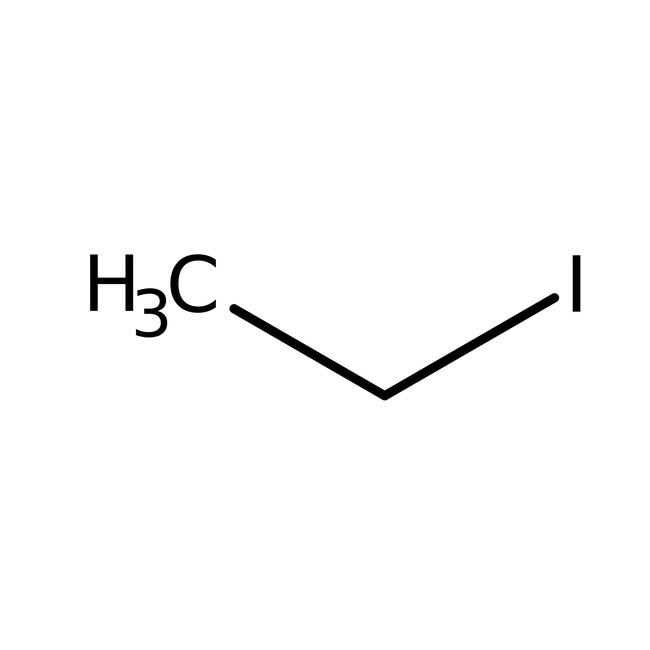
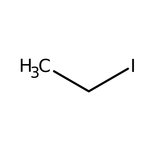
Iodoethane, 98+%, stab. with copper, Thermo Scientific Chemicals
CAS: 75-03-6 | C2H5I | 155.966 g/mol
Catalog number ALFA14444.0E
Quantity:
2500 g
Chemical Identifiers
CAS109-89-7
IUPAC Namediethylamine
Molecular FormulaC4H11N
InChI KeyHPNMFZURTQLUMO-UHFFFAOYSA-N
SMILESCCNCC
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless
Appearance (Form)Liquid
Infrared spectrumConforms
GC>=99.0 %
Water=<0.1 % (K.F.)
View more
Iodoethane is an excellent ethylating agent used in organic synthesis to introduce an ethyl group into a compound. It reacts with magnesium to form the Grignard reagent, ethylmagnesium iodide which is used in organic synthesis. It is involved in the preparation of 1-ethyl-3-nitro-2-phenyl-indole and also serves as hydrogen radical promoter.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Iodoethane is an excellent ethylating agent used in organic synthesis to introduce an ethyl group into a compound. It reacts with magnesium to form the Grignard reagent, ethylmagnesium iodide which is used in organic synthesis. It is involved in the preparation of 1-ethyl-3-nitro-2-phenyl-indole and also serves as hydrogen radical promoter.
Solubility
Miscible with water.
Notes
Moisture and light sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong bases, strong oxidizing agents and magnesium.
Iodoethane is an excellent ethylating agent used in organic synthesis to introduce an ethyl group into a compound. It reacts with magnesium to form the Grignard reagent, ethylmagnesium iodide which is used in organic synthesis. It is involved in the preparation of 1-ethyl-3-nitro-2-phenyl-indole and also serves as hydrogen radical promoter.
Solubility
Miscible with water.
Notes
Moisture and light sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong bases, strong oxidizing agents and magnesium.
RUO – Research Use Only
General References:
- Nijamudheen, A.; Datta, A. Mechanism for C-I Bond Dissociation in Iodoethane, Iodobenzene, and Iodoethene for the C-C Cross Coupling Reactions over Gold Clusters. J. Phys. Chem. C 2013, 117 (41), 21433-21440.
- Ljubic, I.; Matasovic, B.; Bonifacic, M. An efficient buffer-mediated control between free radical substitution and proton-coupled electron transfer: dehalogenation of iodoethane by the alpha-hydroxyethyl radical in aqueous solution. Phys. Chem. Chem. Phys. 2013, 15 (41), 18001-18011.