Search Thermo Fisher Scientific
Thermo Scientific Chemicals
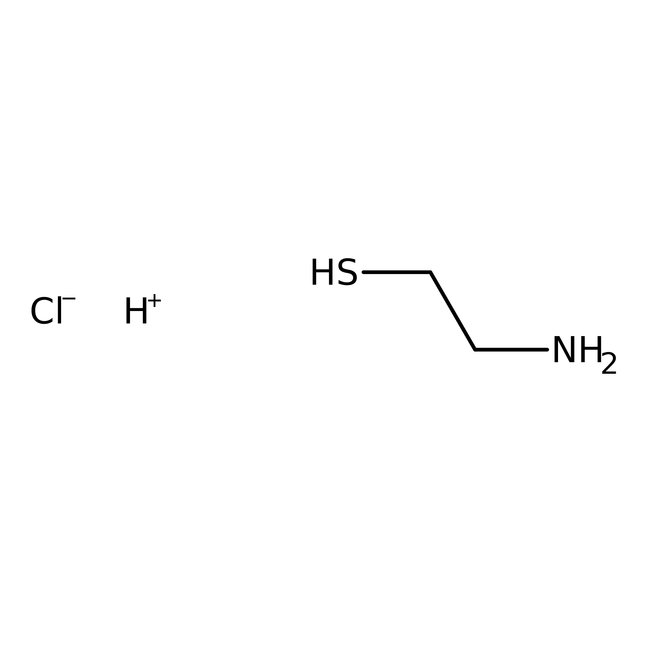
2-Mercaptoethylamine hydrochloride, 97+%, Thermo Scientific Chemicals
CAS: 156-57-0 | C2H8ClNS | 113.60 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA14377.14 | 25 g |
Catalog number ALFA14377.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Specifications
Chemical Name or Material2-Mercaptoethylamine hydrochloride
CAS156-57-0
Health Hazard 1H302-H315-H319-H335
Health Hazard 2GHS H Statement
H302-H315-H319-H335
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H302-H315-H319-H335
Harmful if swallowed.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P261-P264b-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P312-P330-P332+P313-P362-P501c
View more
An inhibitor of DMBA-induced mammary tumors2-Mercaptoethylamine hydrochloride acts as a precursor in taurine biosynthesis and component of coenzyme A. It is involved in the preparation of active pharmaceutical ingredients like ranitidine and nizatidine. It acts as an antidote for acetaminophen poisoning. Further, it is used in the oral treatment of nephropathic cystinosis, radiation sickness and disorders of cysteine excretion. In addition to this, it serves as an antioxidant and an inhibitor of 7,12-Dimethylbenz[a]anthracene (DMBA)-induced tumors.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
An inhibitor of DMBA-induced mammary tumors2-Mercaptoethylamine hydrochloride acts as a precursor in taurine biosynthesis and component of coenzyme A. It is involved in the preparation of active pharmaceutical ingredients like ranitidine and nizatidine. It acts as an antidote for acetaminophen poisoning. Further, it is used in the oral treatment of nephropathic cystinosis, radiation sickness and disorders of cysteine excretion. In addition to this, it serves as an antioxidant and an inhibitor of 7,12-Dimethylbenz[a]anthracene (DMBA)-induced tumors.
Solubility
Soluble in water, ethanol, methanol and dimethyl sulfoxide.
Notes
Store in a cool place. Hygroscopic. Incompatible with strong oxidizing agents.
An inhibitor of DMBA-induced mammary tumors2-Mercaptoethylamine hydrochloride acts as a precursor in taurine biosynthesis and component of coenzyme A. It is involved in the preparation of active pharmaceutical ingredients like ranitidine and nizatidine. It acts as an antidote for acetaminophen poisoning. Further, it is used in the oral treatment of nephropathic cystinosis, radiation sickness and disorders of cysteine excretion. In addition to this, it serves as an antioxidant and an inhibitor of 7,12-Dimethylbenz[a]anthracene (DMBA)-induced tumors.
Solubility
Soluble in water, ethanol, methanol and dimethyl sulfoxide.
Notes
Store in a cool place. Hygroscopic. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- For selective protection of nitrogen by silylation, see: Synthesis, 924 (1980).
- Bhattacharjee, S.; Lee, Y. R.; Ahn, W. S. Post-synthesis functionalization of a zeolitic imidazolate structure ZIF-90: a study on removal of Hg(II) from water and epoxidation of alkenes. CrystEngComm 2015, 17 (12), 2575-2582.
- Yuan, Y. Y.; Du, J. Z.; Wang, J. Two consecutive click reactions as a general route to functional cyclic polyesters. Chem. Commun. 2012, 48 (4), 570-572.