Search Thermo Fisher Scientific
Thermo Scientific Chemicals
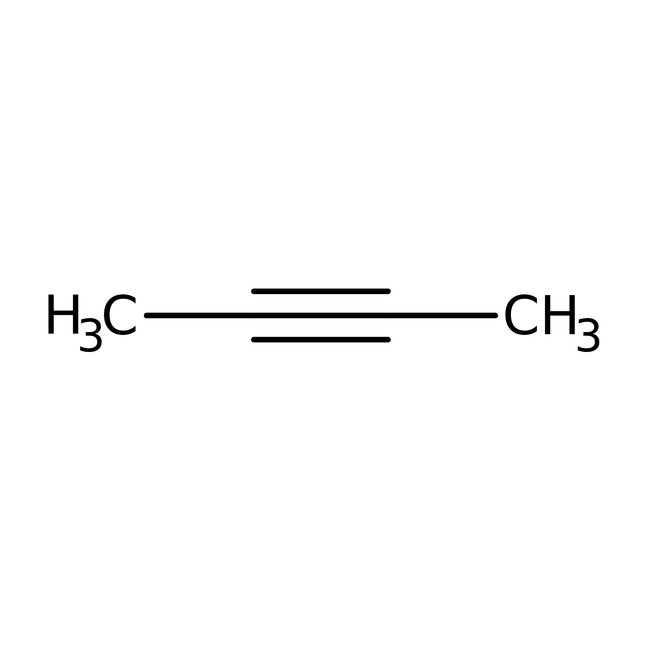
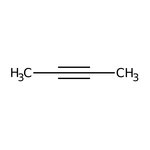
2-Butyne, 98%, Thermo Scientific Chemicals
CAS: 503-17-3 | C4H6 | 54.092 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA13823.22 | 100 g |
Catalog number ALFA13823.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Specifications
Chemical Name or Material2-Butyne
CAS503-17-3
Health Hazard 1H224-H315-H319-H335
Health Hazard 2GHS H Statement
H224-H315-H319-H335
Extremely flammable liquid and vapour.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H224-H315-H319-H335
Extremely flammable liquid and vapour.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P210-P233-P235-P240-P241-P242-P243-P261-P264b-P271-P280-P303+P361+P353-P304+P340-P305+P351+P338-P312-P332+P313-P363-P370+P378q-P501c
View more
2-Butyne is used to synthesize alkylated hydroquinones in the total synthesis of Vitamin E. It is also used as pharmaceutical intermediates.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Butyne is used to synthesize alkylated hydroquinones in the total synthesis of Vitamin E. It is also used as pharmaceutical intermediates.
Solubility
Not miscible in water.
Notes
Avoid extremes of temperature and direct sunlight. Heat-sensitive. Avoid contact with oxidizing materials. Incompatible with Halogens. Chlorinated solvents.
2-Butyne is used to synthesize alkylated hydroquinones in the total synthesis of Vitamin E. It is also used as pharmaceutical intermediates.
Solubility
Not miscible in water.
Notes
Avoid extremes of temperature and direct sunlight. Heat-sensitive. Avoid contact with oxidizing materials. Incompatible with Halogens. Chlorinated solvents.
RUO – Research Use Only
General References:
- Dongmin Shen; Lovat V.C Rees. Adsorption and diffusion of n-butane and 2-butyne in silicalite-I. Zeolites.1991, 11 684-689.
- Kevin C. Wallace; Andy H. Liu; William M. Davis; Richard R. Schrock. Living polymerization of 2-butyne using a well-characterized tantalum catalyst.Organometallics.1989, 8 (3), 644-654.
- With AlCl3, dimerizes to give the complex of the otherwise unstable tetramethylcyclobutadiene, cycloaddition of which with dimethyl acetylenedicarboxylate gives a Dewar benzene: Synthesis, 139 (1971):
- With a catalytic amount of AlCl3, hexamethyl Dewar benzene can be produced directly, with the 2-butyne also acting as the dienophile component: Org. Synth. Coll., 7, 256 (1990).
- Reacts with chlorine to give 3,4-dichloro,1,2,3,4-tetramethylcyclobutene, a precursor of various cyclobutadienyl complexes: Org. Synth. Coll., 5, 370 (1973).
- For use in formation of a Zr metallocycle as an intermediate in the formation of phospholes and analogous heterocycles, see Bis(cyclopentadienyl) zirconium dichloride, 12548.