Search Thermo Fisher Scientific
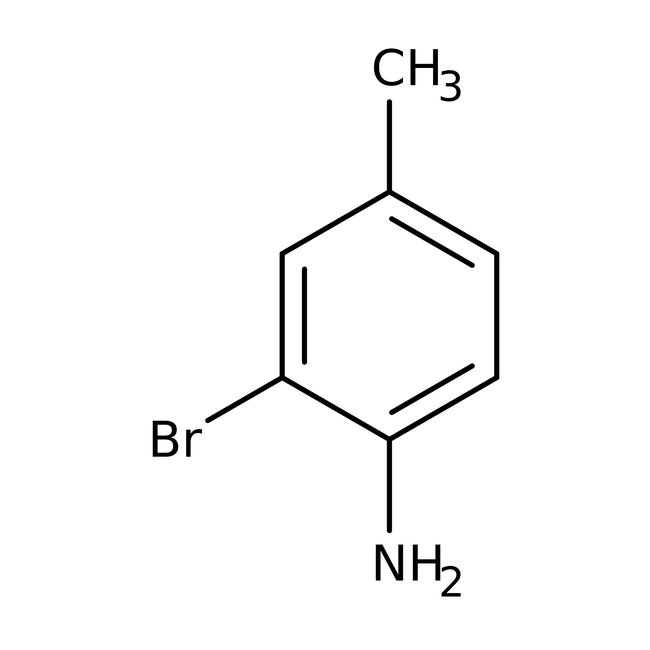
2-Bromo-4-methylaniline, 99%, Thermo Scientific Chemicals
| Catalog Number | Quantity |
|---|---|
| ALFA13818.09 | 10 g |
H301-H311-H315-H319
Toxic if swallowed.
Toxic in contact with skin.
Causes skin irritation.
Causes serious eye irritation.
The N-(3, 5-Dichlorophenyl) naphthaldimine was obtained from the reaction of 2-hy- droxynaphthalene-l-carbaldehyde (0.01 mol) with a solution of 2-bromo-4-methylaniline (0.01 mol) in 40 ml of ethanol. Reaction of 2-bromo-4-methylaniline was quite clean, albeit 3,4-diaminotoluene was isolated just in 37% yield after flash chromatography on silica gel. The bromomethylquinoline starting material 16 was synthesized in two steps using an optimized process route by condensing 2-bromo-4-methylaniline.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
The N-(3, 5-Dichlorophenyl) naphthaldimine was obtained from the reaction of 2-hy- droxynaphthalene-l-carbaldehyde (0.01 mol) with a solution of 2-bromo-4-methylaniline (0.01 mol) in 40 ml of ethanol. Reaction of 2-bromo-4-methylaniline was quite clean, albeit 3,4-diaminotoluene was isolated just in 37% yield after flash chromatography on silica gel. The bromomethylquinoline starting material 16 was synthesized in two steps using an optimized process route by condensing 2-bromo-4-methylaniline.
Solubility
Insoluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents.
General References:
- Renee Aspiotis,; Denis Deschênes,; Daniel Dubé,; Yves Girard,; Zheng Huang,; France Laliberté,; Susana Liu,; Robert Papp,; Donald W. Nicholson,; Robert N. Young.The discovery and synthesis of highly potent subtype selective phosphodiesterase 4D inhibitors. Bioorganic & Medicinal Chemistry Letters 2010, 20(18), 5502-5505.
- A. Elmali,; Y. Elerman,; I. Svoboda and H. Fuess. N-(3,5-Dichlorophenyl)naphthaldimine. Acta Cryst.. 1998, c54 974-976.