Search Thermo Fisher Scientific
Thermo Scientific Chemicals
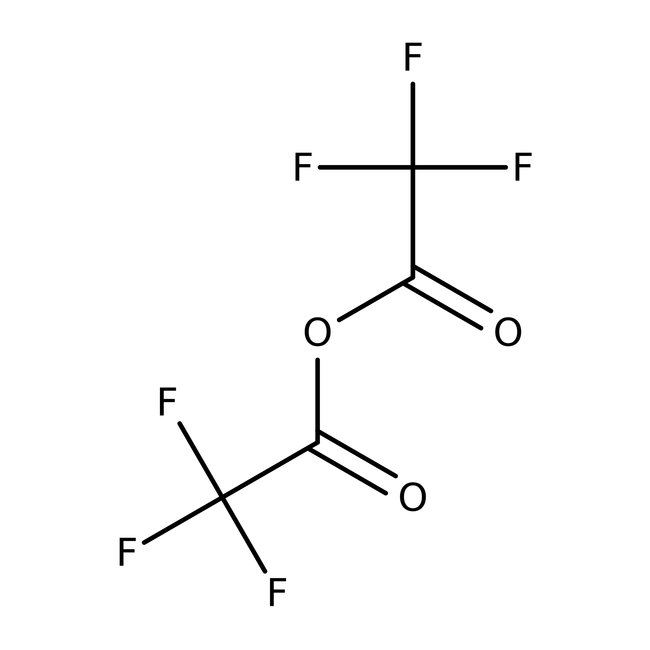
Trifluoroacetic anhydride, 99+%, Thermo Scientific Chemicals
CAS: 407-25-0 | C4F6O3 | 210.03 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA13614.14 | 25 g |
Catalog number ALFA13614.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Chemical Identifiers
CAS7790-69-4
IUPAC Namelithium(1+) nitrate
Molecular FormulaLiNO3
InChI KeyIIPYXGDZVMZOAP-UHFFFAOYSA-N
SMILES[Li+].[O-][N+]([O-])=O
View more
Specifications Specification Sheet
Specification Sheet
Total Metal Impurities0.001% max.
Trifluoroacetic anhydride is used for the introduction of trifluoroacetyl group in organic synthesis. It is involved in the preparation of N- and O-trifluoroacetyl derivatives of a wide range of biologically active compounds for gas chromatography analysis. It plays an important role as desiccant for trifluoroacetic acid. It is also used in the oxidation of aldehydes to acids, esters, amides as well as in the protection of alcohols and amines. In addition, it is used as analytical reagents, solvents and dehydration condensing agent. It serves as an intermediate of fluorine fine chemicals, pharmaceuticals and agrochemicals.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Trifluoroacetic anhydride is used for the introduction of trifluoroacetyl group in organic synthesis. It is involved in the preparation of N- and O-trifluoroacetyl derivatives of a wide range of biologically active compounds for gas chromatography analysis. It plays an important role as desiccant for trifluoroacetic acid. It is also used in the oxidation of aldehydes to acids, esters, amides as well as in the protection of alcohols and amines. In addition, it is used as analytical reagents, solvents and dehydration condensing agent. It serves as an intermediate of fluorine fine chemicals, pharmaceuticals and agrochemicals.
Solubility
Miscible with benzene, dichloromethane, diethyl ether, dimethylformamide, terahydrofuran and acetonitrile.
Notes
Hygroscopic and moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents, strong bases, strong acids, and alcohols.
Trifluoroacetic anhydride is used for the introduction of trifluoroacetyl group in organic synthesis. It is involved in the preparation of N- and O-trifluoroacetyl derivatives of a wide range of biologically active compounds for gas chromatography analysis. It plays an important role as desiccant for trifluoroacetic acid. It is also used in the oxidation of aldehydes to acids, esters, amides as well as in the protection of alcohols and amines. In addition, it is used as analytical reagents, solvents and dehydration condensing agent. It serves as an intermediate of fluorine fine chemicals, pharmaceuticals and agrochemicals.
Solubility
Miscible with benzene, dichloromethane, diethyl ether, dimethylformamide, terahydrofuran and acetonitrile.
Notes
Hygroscopic and moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents, strong bases, strong acids, and alcohols.
RUO – Research Use Only
General References:
- Trifluoroacetylating agent for protection of alcohols and amines: Helv. Chim. Acta., 37, 443 (1954); Tetrahedron, 43, 5583 (1987); Tetrahedron Lett., 28, 4737 (1987), useful, e.g. as volatile derivatives in GC: J. Chromat., 43, 129 (1969); 61, 225 (1971); 93, 223, 447 (1974); D. R. Knapp, Handbook of Analytical Derivatisation Reactions, Wiley, N.Y. (1979); Handbook of Derivatives for Chromatography, 2nd ed., K. Blau and J. M. Halket, Eds., Wiley, Chichester (1993).
- Trifluoroacetyl esters are rapidly hydrolyzed at pH 7: Tetrahedron Lett., 1039 (1963). Trifluoroacetamides are also readily hydrolyzed e.g. by mild base; selective cleavage of a trifluoroacetamide can be achieved in the presence of a methyl ester: J. Org. Chem., 54, 2498 (1989). For the racemization-free removal of the N-trifluoroacetyl group from peptides by NaBH4, see: Chem. Ber., 103, 2437 (1970).
- In pyridine and dichloromethane or ether, converts acid chlorides to trifluoromethyl ketones in good yields via a trifluoroacyl ketene intermediate: Tetrahedron, 51, 2573 (1995). The method has been extended to the synthesis of a range of trifluoromethylated heterocycles: Tetrahedron, 51, 2585 (1995):
- Mild conversion of aryl halides to aryl trifluoromethyl ketones via Pd catalyzed stannylation: Synlett, 165 (1995).
- In pyridine, dehydrates carboxamides or aldoximes to nitriles: Tetrahedron Lett., 1813 (1977); Synthesis, 56, (1979). With triethylamine, effects the dehydration of aldols to enones, where other methods are less successful: Org. Synth. Coll., 8, 210 (1993).
- For the ɑ-trifluoroacetylation of a phosphonium salt in a route to perfluoroalkyl acetylenes, see: Org. Synth. Coll., 9, 436 (1998). For reaction scheme, see (Ethoxycarbonyl methyl) triphenyl phosphonium bromide, A16347.