Search Thermo Fisher Scientific
Thermo Scientific Chemicals
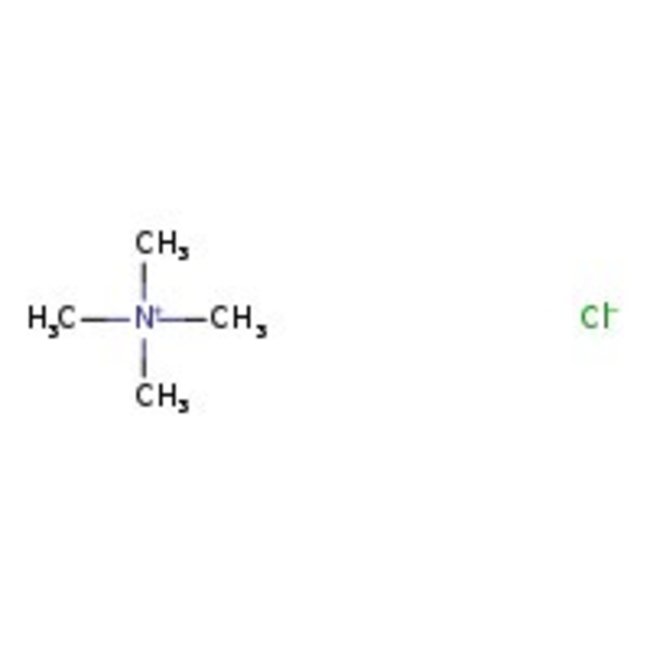
Tetramethylammonium chloride, 97%, Thermo Scientific Chemicals
CAS: 75-57-0 | C4H12ClN | 109.597 g/mol
Catalog number ALFA13288.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Specifications
Chemical Name or MaterialTetramethylammonium chloride
CAS75-57-0
Health Hazard 1H300-H311-H315-H319-H370
Health Hazard 2GHS H Statement
H301-H311-H315-H319-H335
Toxic if swallowed.
Toxic in contact with skin.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H301-H311-H315-H319-H335
Toxic if swallowed.
Toxic in contact with skin.
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3P260-P264b-P270-P280-P301+P310-P302+P352-P305+P351+P338-P307+P311-P312-P330-P332+P313-P361-P363-P501c
View more
Tetramethylammonium binds AT-rich DNA polymers while concomitantly abolishing the preferential melting of AT versus GC base pairs. Supplied as 0.2 ?m filtered solution in 18 megohm water. It is employed as a phase transfer catalyst.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Tetramethylammonium binds AT-rich DNA polymers while concomitantly abolishing the preferential melting of AT versus GC base pairs. Supplied as 0.2 μm filtered solution in 18 megohm water. It is employed as a phase transfer catalyst.
Solubility
Soluble in water. Insoluble in ether, and benzene.
Notes
Hygroscopic. Store at room temperature. Incompatible with oxidizing agents. Ensure adequate ventilation.
Tetramethylammonium binds AT-rich DNA polymers while concomitantly abolishing the preferential melting of AT versus GC base pairs. Supplied as 0.2 μm filtered solution in 18 megohm water. It is employed as a phase transfer catalyst.
Solubility
Soluble in water. Insoluble in ether, and benzene.
Notes
Hygroscopic. Store at room temperature. Incompatible with oxidizing agents. Ensure adequate ventilation.
RUO – Research Use Only
General References:
- J. Kathleen Buckner.; William L. Jorgensen. Energetics and hydration of the constituent ion pairs of tetramethylammonium chloride. J. Am. Chem. Soc. 1989, 111 (7),2507-2516 .
- Anthony G. Dilella.; Savio L.C. Woo. [49] Hybridization of genomic DNA to oligonucleotide probes in the presence of tetramethylammonium chloride.Methods Enzymol. 1987, 152 447-451 .
- Thermally-stable phase-transfer catalyst (see Appendix 2) which has been used in nucleophilic displacement reactions of aryl nitro groups with KF in tetramethylene sulfone: J. Org. Chem., 56, 6406 (1991). A detailed study of the halex Cl - /F - exchange reaction of activated aryl chlorides, for example the conversion of 1,2-dichloro-4-nitrobenzene to 2-chloro-1-fluoro-4-nitrobenzene in DMSO at 120°C showed tetramethylammonium chloride to be superior to other common phase-transfer catalysts. It was necessary to pre-dry the catalyst to <0.2% water to avoid competition from hydrolytic displacement: Chem. Commun., 297 (1996).
- Compare also Tetraphenyl phosphonium bromide, A15860 and Tetraphenyl phosphonium chloride, A10575.