Search Thermo Fisher Scientific
Thermo Scientific Chemicals
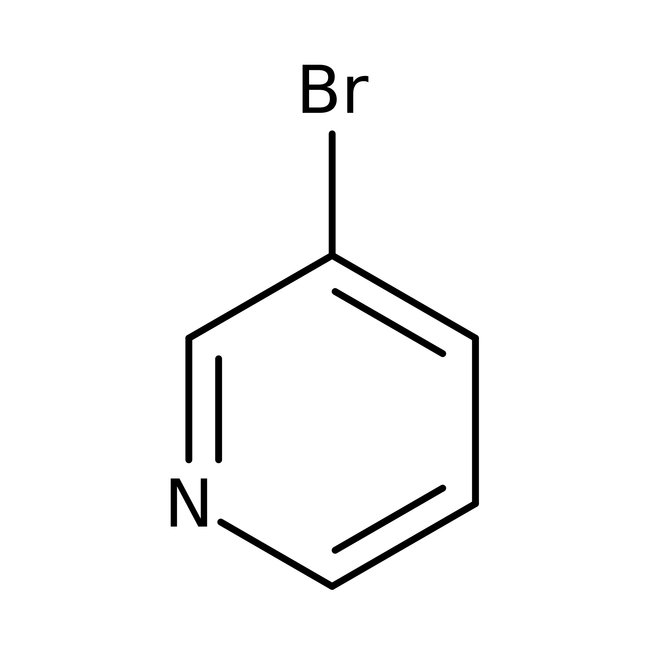
3-Bromopyridine, 98+%, Thermo Scientific Chemicals
CAS: 626-55-1 | C5H4BrN | 158.00 g/mol
Catalog number ALFA12894.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS5470-11-1
IUPAC Namehydroxylamine hydrochloride
Molecular FormulaClH4NO
InChI KeyWTDHULULXKLSOZ-UHFFFAOYSA-N
SMILESCl.NO
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White
Appearance (Form)Crystalline powder or crystals and/or chunks
Titration with NaOH99.0 to 101.0 %
Infrared spectrumConforms
Sulfate (SO4)=<0.005 %
View more
3-Bromopyridine undergoes lithiation with LDA at low temperatures in the 4-position: Heterocycles, involved in formation of the Grignard by Mg exchange with i-PrMgCl and in transmetallation of the lithiated derivative with zinc chloride followed by treatment with an electrophile as a route to 4-substituted 3-bromopyridines.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
3-Bromopyridine undergoes lithiation with LDA at low temperatures in the 4-position: Heterocycles, involved in formation of the Grignard by Mg exchange with i-PrMgCl and in transmetallation of the lithiated derivative with zinc chloride followed by treatment with an electrophile as a route to 4-substituted 3-bromopyridines.
Solubility
Soluble in water (31 g/L at 20°C).
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are opened must be carefully resealed and kept upright to prevent leakage. Incompatible with strong acids.
3-Bromopyridine undergoes lithiation with LDA at low temperatures in the 4-position: Heterocycles, involved in formation of the Grignard by Mg exchange with i-PrMgCl and in transmetallation of the lithiated derivative with zinc chloride followed by treatment with an electrophile as a route to 4-substituted 3-bromopyridines.
Solubility
Soluble in water (31 g/L at 20°C).
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are opened must be carefully resealed and kept upright to prevent leakage. Incompatible with strong acids.
RUO – Research Use Only
General References:
- Jennifer A. Love Dr.; John P. Morgan.;Tina M. Trnka.;Robert H. Grubbs Prof. A Practical and Highly Active Ruthenium-Based Catalyst that Effects the Cross Metathesis of Acrylonitrile. Angewandte Chemie International Edition. 2002, 41 (21), 4035-4037.
- Wenjie Li.; Dorian P. Nelson.; Mark S. Jensen.; R. Scott Hoerrner.; Dongwei Cai.; Robert D. Larsen.; Paul J. Reider. An Improved Protocol for the Preparation of 3-Pyridyl- and Some Arylboronic Acids. J. Org. Chem. 2002, 57 (16), 5394-5397.
- Undergoes lithiation with LDA at low temperatures in the 4-position: Heterocycles, 35, 151 (1993). Formation of the Grignard by Mg exchange with i-PrMgCl: Tetrahdron Lett., 40, 4339 (1999). Transmetallation of the lithiated derivative with ZnCl2 followed by treatment with an electrophile has been described, as a route to 4-substituted 3-bromopyridines: Org. Lett., 3, 835 (2001).