Search Thermo Fisher Scientific
Thermo Scientific Chemicals
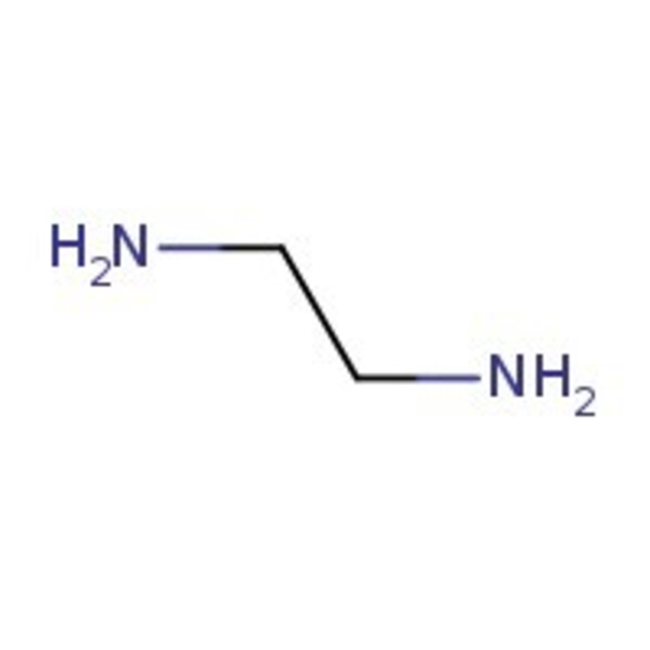
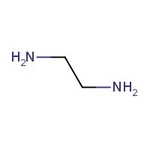
Ethylenediamine, 99%, Thermo Scientific Chemicals
Strongly basic amine useful as a building block in chemical synthesis
Catalog number ALFA12132.0F
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
2500 mL
Specifications
Chemical Name or MaterialEthylenediamine
CAS107-15-3
Health Hazard 1H226-H302+H332-H311-H314-H317-H334-H335
Health Hazard 2GHS H Statement
H334-H314-H226-H302-H312-H317
May cause allergy or asthma symptoms or breathing difficulties if inhaled.
Causes severe skin burns and eye damage.
Flammable liquid and vapor.
Harmful if swallowed.
Harmful in contact with skin.
May cause an allergic skin reaction.
H334-H314-H226-H302-H312-H317
May cause allergy or asthma symptoms or breathing difficulties if inhaled.
Causes severe skin burns and eye damage.
Flammable liquid and vapor.
Harmful if swallowed.
Harmful in contact with skin.
May cause an allergic skin reaction.
Health Hazard 3P210-P233-P235-P240-P241-P242-P243-P260-P264b-P270-P271-P272-P280-P285-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P333+P313-P363-P370+P378q-P501c
View more
Ethylenediamine is a strongly basic amine useful as a building block in chemical synthesis. It is used as a solvent to dissolve proteins such as albumins and casein. It is widely used for color photography developers, binders, adhesives, fabric softeners, curing agents for epoxys and dyes. As a corrosion inhibitor, it plays a vital role in paints and coolants. It is used as an intermediate in the preparation of polyamide resins, fuel additives and lubricants. It acts as a precursor for many polymers like polyurethane fibers and poly(amidoamine), ethylenediamine dihydroiodide (EDDI) as well as the bleaching activator, tetraacetylethylenediamine. It is an important chelating ligand used in the preparation of coordination compounds viz. tris(ethylenediamine)cobalt(III) chloride. It is also involved in the manufacture of many industrial chemicals and forms derivatives with carboxylic acids, nitriles, alcohols, alkylating agents, carbon disulfide, aldehydes and ketones. It is a basic building block to prepare heterocyclic compound such as imidazolidines.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Ethylenediamine is a strongly basic amine useful as a building block in chemical synthesis. It is used as a solvent to dissolve proteins such as albumins and casein. It is widely used for color photography developers, binders, adhesives, fabric softeners, curing agents for epoxys and dyes. As a corrosion inhibitor, it plays a vital role in paints and coolants. It is used as an intermediate in the preparation of polyamide resins, fuel additives and lubricants. It acts as a precursor for many polymers like polyurethane fibers and poly(amidoamine), ethylenediamine dihydroiodide (EDDI) as well as the bleaching activator, tetraacetylethylenediamine. It is an important chelating ligand used in the preparation of coordination compounds viz. tris(ethylenediamine)cobalt(III) chloride. It is also involved in the manufacture of many industrial chemicals and forms derivatives with carboxylic acids, nitriles, alcohols, alkylating agents, carbon disulfide, aldehydes and ketones. It is a basic building block to prepare heterocyclic compound such as imidazolidines.
Notes
Air and moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with oxidizing agents, phosphorus halides, aldehydes and organic halides.
Ethylenediamine is a strongly basic amine useful as a building block in chemical synthesis. It is used as a solvent to dissolve proteins such as albumins and casein. It is widely used for color photography developers, binders, adhesives, fabric softeners, curing agents for epoxys and dyes. As a corrosion inhibitor, it plays a vital role in paints and coolants. It is used as an intermediate in the preparation of polyamide resins, fuel additives and lubricants. It acts as a precursor for many polymers like polyurethane fibers and poly(amidoamine), ethylenediamine dihydroiodide (EDDI) as well as the bleaching activator, tetraacetylethylenediamine. It is an important chelating ligand used in the preparation of coordination compounds viz. tris(ethylenediamine)cobalt(III) chloride. It is also involved in the manufacture of many industrial chemicals and forms derivatives with carboxylic acids, nitriles, alcohols, alkylating agents, carbon disulfide, aldehydes and ketones. It is a basic building block to prepare heterocyclic compound such as imidazolidines.
Notes
Air and moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with oxidizing agents, phosphorus halides, aldehydes and organic halides.
RUO – Research Use Only
General References:
- The Li derivative in excess amine is a strong base for the rearrangement of primary allylic alcohols to the isomeric aldehydes in high yield: J. Chem. Soc., Chem. Commun., 812 (1985).
- At high temperatures, reduces nitroarenes to azo-compounds. Reaction does not occur for nitro-compounds with o-substituents or p-amino-substituents: J. Org. Chem., 49, 1215 (1984).
- The ethylenediamine complex of Cr2+ reduces aryl bromides to the hydrocarbons. For an example, see: Org. Synth. Coll., 6, 821 (1988).
- Xue, B.; Zhu, J.; Liu, N.; Li, Y. Facile functionalization of graphene oxide with ethylenediamine as a solid base catalyst for Knoevenagel condensation reaction. Catal. Commun. 2015, 64, 105-109.
- Moradpour, A.; Ghaffarinejad, A.; Maleki, A.; Eskandarpour, V.; Motaharian, A. Low loaded palladium nanoparticles on ethylenediamine-functionalized cellulose as an efficient catalyst for electrochemical hydrogen production. RSC Adv. 2015, 5 (86), 70668-70674.