Search Thermo Fisher Scientific
Thermo Scientific Chemicals
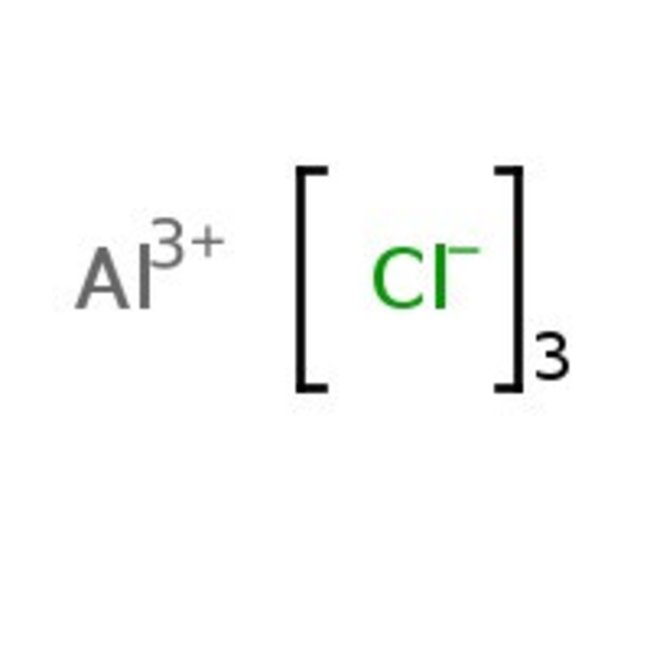
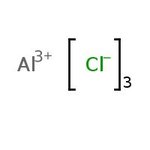
Aluminum chloride, anhydrous, granular, 99%, Thermo Scientific Chemicals
Catalog number ALFA11892.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Chemical Identifiers
CAS10035-06-0
IUPAC Namebismuth(3+) pentahydrate trinitrate
Molecular FormulaBiH10N3O14
InChI KeyFBXVOTBTGXARNA-UHFFFAOYSA-N
SMILESO.O.O.O.O.[Bi+3].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White
Total trace metal impurities=<0.001 %
Appearance (Form)Crystals
Aluminum chloride is used as a catalyst for Friedel-Crafts acylation and alkylation of aromatic compounds. It is one of the most commonly employed Lewis acids for a wide variety of organic transformations. It catalyzes the ene reaction, polymerization and isomerization reactions. For example, it can be used for the synthesis of ethyl benzene which is a precursor for producing polystyrene. It can be used for producing dodecylbenzene, a key intermediate for detergents. It is useful in the production of anthroquinone, the presursor for dyestuffs. It is used in the synthesis of bis (arene) metal complexes, through Fischer-Hafner synthesis. Gattermann-Koch reaction employs aluminum chloride for introducing formyl group onto aromatic rings.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Aluminum chloride is used as a catalyst for Friedel-Crafts acylation and alkylation of aromatic compounds. It is one of the most commonly employed Lewis acids for a wide variety of organic transformations. It catalyzes the ene reaction, polymerization and isomerization reactions. For example, it can be used for the synthesis of ethyl benzene which is a precursor for producing polystyrene. It can be used for producing dodecylbenzene, a key intermediate for detergents. It is useful in the production of anthroquinone, the presursor for dyestuffs. It is used in the synthesis of bis (arene) metal complexes, through Fischer-Hafner synthesis. Gattermann-Koch reaction employs aluminum chloride for introducing formyl group onto aromatic rings.
Solubility
Soluble in water, hydrochloric acid and ethanol. Slightly soluble in benzene.
Notes
Moisture sensitive. Reacts violently with water with liberation of heat.
Aluminum chloride is used as a catalyst for Friedel-Crafts acylation and alkylation of aromatic compounds. It is one of the most commonly employed Lewis acids for a wide variety of organic transformations. It catalyzes the ene reaction, polymerization and isomerization reactions. For example, it can be used for the synthesis of ethyl benzene which is a precursor for producing polystyrene. It can be used for producing dodecylbenzene, a key intermediate for detergents. It is useful in the production of anthroquinone, the presursor for dyestuffs. It is used in the synthesis of bis (arene) metal complexes, through Fischer-Hafner synthesis. Gattermann-Koch reaction employs aluminum chloride for introducing formyl group onto aromatic rings.
Solubility
Soluble in water, hydrochloric acid and ethanol. Slightly soluble in benzene.
Notes
Moisture sensitive. Reacts violently with water with liberation of heat.
RUO – Research Use Only
General References:
- Shilina, M. I.; Vasilevskii, G. Y.; Rostovshchikova, T. N.; Murzin, V. Y. Unusual coordination state of cobalt ions in zeolites modified by aluminum chloride. Dalton Trans. 2015, 44 (29), 13282-13293.
- Azarniya, A.; Azarniya, A.; Hosseini, H. R. M.; Simch, A. Nanostructured aluminum titanate (Al2TiO5) particles and nanofibers: Synthesis and mechanism of microstructural evolution. Mater. Charact. 2015, 103, 125-132.

.png-150.jpg)

