Search Thermo Fisher Scientific
Thermo Scientific Chemicals
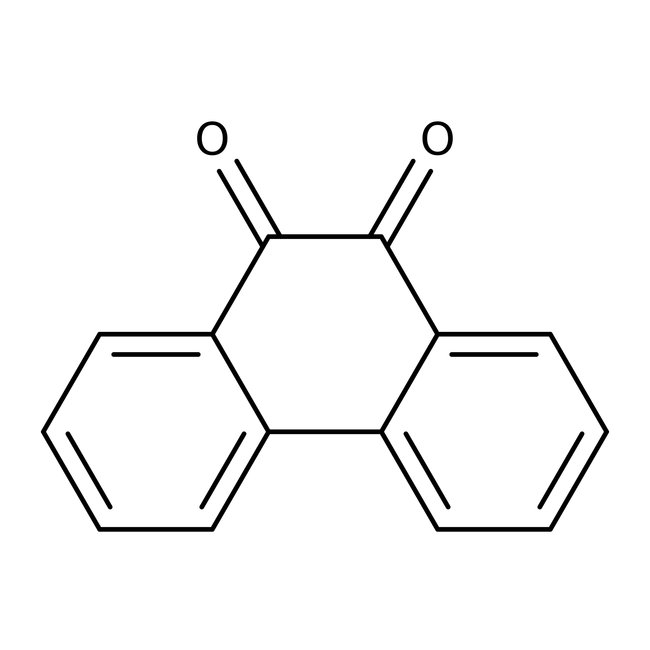
9,10-Phenanthrenequinone, 95%, Thermo Scientific Chemicals
CAS: 84-11-7 | C14H8O2 | 208.216 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA11762.06 | 5 g |
Catalog number ALFA11762.06
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
5 g
Specifications
Chemical Name or Material9,10-Phenanthrenequinone
CAS84-11-7
Health Hazard 1H315-H319-H335
Health Hazard 2GHS H Statement
H319
Causes serious eye irritation.
H319
Causes serious eye irritation.
Health Hazard 3P261-P264b-P271-P280-P302+P352-P304+P340-P305+P351+P338-P312-P332+P313-P362-P501c
View more
9,10-Phenanthrenequinon may be used for high quality passivation on silicon (100) surfaces. Quinones may serve as substrates for a variety of flavoenzymes, useful fluorescent compound and quinone. Also used in dyes, as a hardener for dental restoration, for the determination of copper by spectrophotometric method, and for organic synthesis.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
9,10-Phenanthrenequinon may be used for high quality passivation on silicon (100) surfaces. Quinones may serve as substrates for a variety of flavoenzymes, useful fluorescent compound and quinone. Also used in dyes, as a hardener for dental restoration, for the determination of copper by spectrophotometric method, and for organic synthesis.
Solubility
Insoluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from oxidizing agent.
9,10-Phenanthrenequinon may be used for high quality passivation on silicon (100) surfaces. Quinones may serve as substrates for a variety of flavoenzymes, useful fluorescent compound and quinone. Also used in dyes, as a hardener for dental restoration, for the determination of copper by spectrophotometric method, and for organic synthesis.
Solubility
Insoluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from oxidizing agent.
RUO – Research Use Only
General References:
- Avasthi, Sushobhan, et al. Electronic structure and band alignment of 9, 10-phenanthrenequinone passivated silicon surfaces. Surface Science., 2011, 605 (13), 1308-1312.
- P L Chesis; D E Levin; M T Smith; L Ernster; B N Ames. Mutagenicity of quinones: pathways of metabolic activation and detoxification. PNAS, 1984, 81 (6), 1696-1700.
- Specific, highly sensitive reagent for arginine and arginyl peptides: Biochem. Biophys. Acta, 130, 538 (1966).
- Reagent for dehydrogenation, e.g. of thiazolines to thiazoles in high yield: J. Chem. Soc.(C), 1061 (1966). Review: Chem. Rev., 78, 317 (1978).
- Like 1,2-Cyclohexanedione, A14401, has been used by Ley's group for protection of trans-1,2-diols as cyclic diacetals. The method is particularly applicable to the carbohydrate field: Synlett, 793 (1996); J. Chem. Soc., Perkin 1, 2023 (1997):