Search Thermo Fisher Scientific
Thermo Scientific Chemicals
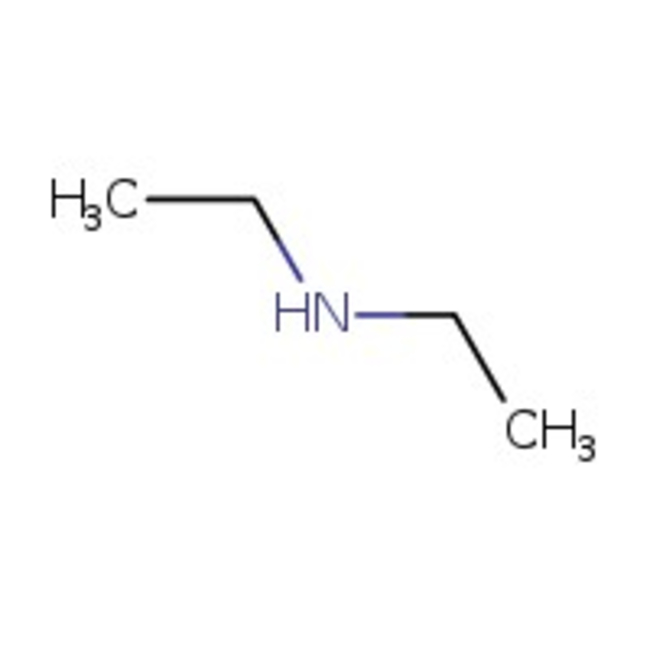
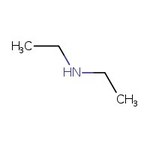
Diethylamine, 99+%, Thermo Scientific Chemicals
| Catalog Number | Quantity |
|---|---|
| ALFA11716.0F | 2500 mL |
Catalog number ALFA11716.0F
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
2500 mL
Chemical Identifiers
CAS1309-48-4
IUPAC Nameoxomagnesium
Molecular FormulaMgO
InChI KeyCPLXHLVBOLITMK-UHFFFAOYSA-N
SMILESO=[Mg]
View more
Specifications Specification Sheet
Specification Sheet
Particle size=<1 % Residue on 150 µm (>100 mesh) sieve
Diethylamine is used as a corrosion inhibitor. It is also used in the production of rubber, resins, dyes and pharmaceuticals.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Diethylamine is used as a corrosion inhibitor. It is also used in the production of rubber, resins, dyes and pharmaceuticals. It is also used to cook lysergic acid diethylamide (LSD) and is strictly watched by Drug Enforcement Administration (DEA).
Solubility
Miscible with water, alcohol, ether, chloroform and carbon terachloride.
Notes
Air sensitive. Incompatible with strong oxidizing agents.
Diethylamine is used as a corrosion inhibitor. It is also used in the production of rubber, resins, dyes and pharmaceuticals. It is also used to cook lysergic acid diethylamide (LSD) and is strictly watched by Drug Enforcement Administration (DEA).
Solubility
Miscible with water, alcohol, ether, chloroform and carbon terachloride.
Notes
Air sensitive. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Alkali metal diethylamides, generated with n-BuLi or NaH, have been used for the cleavage of ethers: J. Organomet. Chem., 55, 41 (1973); Synthesis, 638 (1980), and of alkyl aryl sulfides, for which HBr and HI are usually poor: Synthesis, 583 (1982).
- In combination with Lithium aluminum hydride, A18116, generates lithium tris(diethylamino)aluminum hydride, which is a useful selective reducing agent, for example: esters to aldehydes: J. Org. Chem., 52, 5486 (1987); amides to aldehydes: Tetrahedron Lett., 32, 6903 (1991).
- Wang, N. N.; Zhang, D. L.; Jiang, X. H. Detection of Xeljanz Enantiomers in Diethyl Amine Active Pharmaceutical Ingredients and Tablets. Chirality 2015, 27 (3), 235-238.
- Higashino, T.; Soya, T.; Kim, W.; Kim, D.; Osuka, A. A Mobius Aromatic [28]Hexaphyrin Bearing a Diethylamine Group: A Rigid but Smooth Conjugation Circuit. Angew. Chem. Int. Ed. 2015, 54 (18), 5456-5459.