Search Thermo Fisher Scientific
Thermo Scientific Chemicals
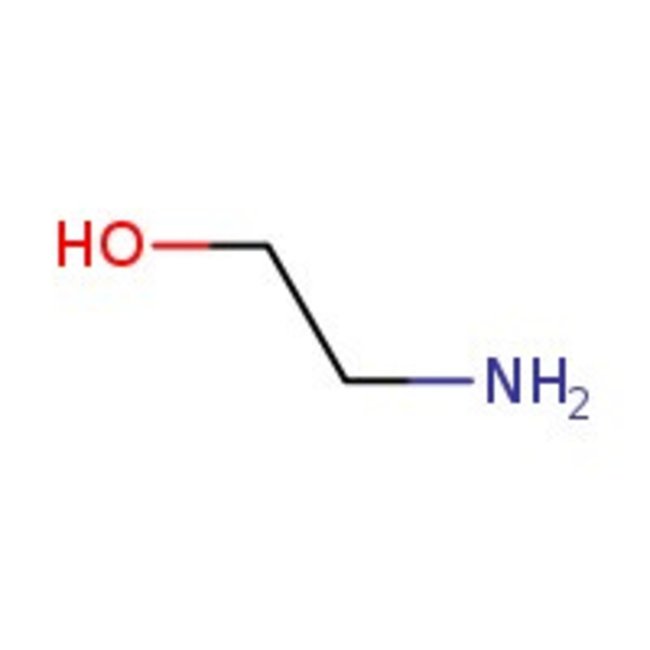
Ethanolamine, 98+%, Thermo Scientific Chemicals
Catalog number ALFA11697.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Chemical Name or MaterialEthanolamine
CAS141-43-5
Health Hazard 1H227-H302+H312+H332-H314-H335
Health Hazard 2GHS H Statement
H314-H302-H312-H332-H227
Causes severe skin burns and eye damage.
Harmful if swallowed.
Harmful in contact with skin.
Harmful if inhaled.
Combustible liquid.
H314-H302-H312-H332-H227
Causes severe skin burns and eye damage.
Harmful if swallowed.
Harmful in contact with skin.
Harmful if inhaled.
Combustible liquid.
Health Hazard 3P210-P235-P260-P264b-P270-P271-P280-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P363-P370+P378q-P501c
View more
Ethanolamine is also used in messenger molecules such as palmitoylethanolamide, which have an effect on CB1 receptors. Ethanolamine is used as a scrubber for the removal of methyl bromide. Polyaniline doping ethanolamine is used for modified electrode preparation to measure uric acid in human body with the presence of antiscorbutic acid.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Ethanolamine is also used in messenger molecules such as palmitoylethanolamide, which have an effect on CB1 receptors. Ethanolamine is used as a scrubber for the removal of methyl bromide. Polyaniline doping ethanolamine is used for modified electrode preparation to measure uric acid in human body with the presence of antiscorbutic acid.
Solubility
Miscible with water.
Notes
Store it away from heat, spark, open flames, hot surfaces and smoke. The storage area should be safe and enclosed.
Ethanolamine is also used in messenger molecules such as palmitoylethanolamide, which have an effect on CB1 receptors. Ethanolamine is used as a scrubber for the removal of methyl bromide. Polyaniline doping ethanolamine is used for modified electrode preparation to measure uric acid in human body with the presence of antiscorbutic acid.
Solubility
Miscible with water.
Notes
Store it away from heat, spark, open flames, hot surfaces and smoke. The storage area should be safe and enclosed.
RUO – Research Use Only
General References:
- Reagent for the demethylation of quaternary ammonium salts formed by the exhaustive methylation of aromatic amines, providing a route to N,N-dimethyl arylamines: Org. Synth. Coll., 5, 1018 (1973).
- Base for the cleavage of Fmoc protecting groups: J. Am. Chem. Soc., 92, 5748 (1970); J. Org. Chem., 37, 3404 (1972).
- Chen, Y.; Hu, Z.; Zhong, Z.; Shi, W.; Peng, J.; Wang, J.; Cao, Y. Aqueous Solution Processed, Ultrathin ZnO Film with Low Conversion Temperature as the Electron Transport Layer in the Inverted Polymer Solar Cells. J. Phys. Chem. C 2014, 118 (38), 21819-21825.
- Hettenbach, K.; Ende, D. J.; Leeman, K.; Dias, E.; Kasthurikrishnan, N.; Brenek, S. J.; Ahlijanian, P. Development and Scale-Up of an Aqueous Ethanolamine Scrubber for Methyl Bromide Removal. Org. Proc. Res. Dev. 2002, 6 (4), 407-415.