Search Thermo Fisher Scientific
Thermo Scientific Chemicals
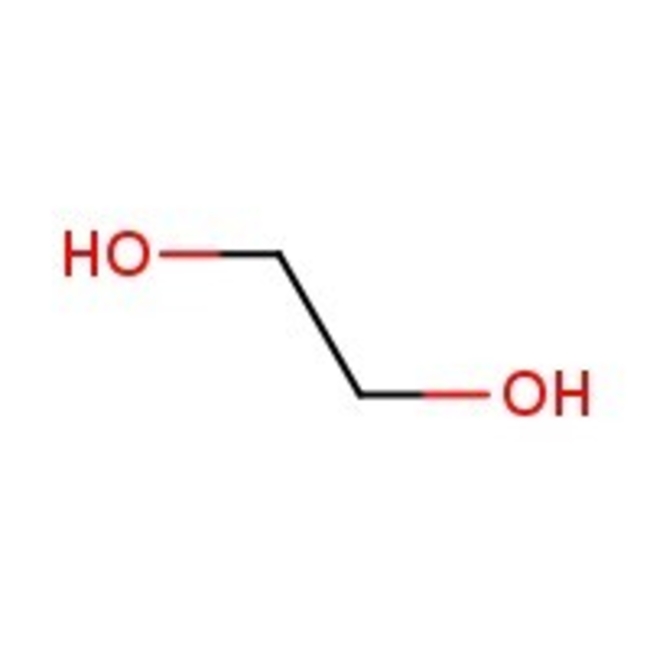
Ethylene glycol, 99%, Thermo Scientific Chemicals
CAS: 107-21-1 | C2H6O2 | 62.068 g/mol
Catalog number ALFA11591.0E
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
2500 g
Chemical Identifiers
CAS139042-59-4
IUPAC Name1-(6-bromopyridin-3-yl)ethan-1-one
Molecular FormulaC7H6BrNO
InChI KeyMUKKGHQBUKOMTD-UHFFFAOYSA-N
SMILESCC(=O)C1=CN=C(Br)C=C1
View more
Specifications Specification Sheet
Specification Sheet
Assay (GC)≥96.0%
FormCrystals or powder or crystalline powder
Water Content (Karl Fischer Titration)≤1.5%
Appearance (Color)Cream to pale yellow
Melting Point (clear melt)122.0-128.0°C
Ethylene glycol is used as a precursor for the syntheses of polymers like polyester fibers, resins and polyethylene terephthalate, which finds application in making plastic bottles. It acts as an intermediate in the synthesis of 1,4-dioxane. It plays an important role in organic synthesis as a protecting group for carbonyl groups. It is a useful desiccant due to its high boiling point and affinity towards water. It plays an important role to remove water vapor from natural gas before proceeding for further processes. Its major application is as a medium for convective heat transfer in automobiles and liquid cooled computers.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Ethylene glycol is used as a precursor for the syntheses of polymers like polyester fibers, resins and polyethylene terephthalate, which finds application in making plastic bottles. It acts as an intermediate in the synthesis of 1,4-dioxane. It plays an important role in organic synthesis as a protecting group for carbonyl groups. It is a useful desiccant due to its high boiling point and affinity towards water. It plays an important role to remove water vapor from natural gas before proceeding for further processes. Its major application is as a medium for convective heat transfer in automobiles and liquid cooled computers.
Solubility
Miscible with water, toluene, tetrahydrofuran, alcohol, chloroform and other organic solvents.
Notes
Hygroscopic. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong acids, strong oxidizing agents, strong bases, aldehydes and aluminum.
Ethylene glycol is used as a precursor for the syntheses of polymers like polyester fibers, resins and polyethylene terephthalate, which finds application in making plastic bottles. It acts as an intermediate in the synthesis of 1,4-dioxane. It plays an important role in organic synthesis as a protecting group for carbonyl groups. It is a useful desiccant due to its high boiling point and affinity towards water. It plays an important role to remove water vapor from natural gas before proceeding for further processes. Its major application is as a medium for convective heat transfer in automobiles and liquid cooled computers.
Solubility
Miscible with water, toluene, tetrahydrofuran, alcohol, chloroform and other organic solvents.
Notes
Hygroscopic. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong acids, strong oxidizing agents, strong bases, aldehydes and aluminum.
RUO – Research Use Only
General References:
- For examples of use in acetal (1,3-dioxolane) formation, catalyzed by tosic acid with azeotropic water removal, see: Org. Synth. Coll., 6, 567 (1988); 7, 241, 271 (1990). A mild dehydrating agent, e.g. triethyl orthoformate may be used as an alternative: J. Org. Chem., 48, 2122 (1983). PPTS (Pyridinium p-toluenesulfonate, A15708) has been recommended as a catalyst for the formation and cleavage of ethylene acetals, being less likely to cause acid-catalyzed rearrangements than tosic acid itself: Synthesis, 724 (1979). For acetalization of ɑß-enals, tartaric acid in the presence of anhydrous MgSO4 gives good results: J. Org. Chem., 60, 2931 (1995). Review of the preparation of acetals: Synthesis, 501 (1981). See also 2,2-Dimethyl-1,3-dioxolane, L00482.
- In combination with TMS chloride, ɑß-enones are converted to ß-chloro acetals in one step: Tetrahedron Lett., 25, 3805 (1984).
- Pluta, M.; Piorkowska, E. Tough and transparent blends of polylactide with block copolymers of ethylene glycol and propylene glycol. Polym. Test. 2015, 41, 209-218.
- Zahmatkesh, H. G,, Javanbakht, M.; Ghaemi, M. Ethylene glycol-assisted hydrothermal synthesis and characterization of bow-tie-like lithium iron phosphate nanocrystals for lithium-ion batteries. J. Power Sources 2015, 284, 339-348.

.png-150.jpg)
