Search Thermo Fisher Scientific
Thermo Scientific Chemicals
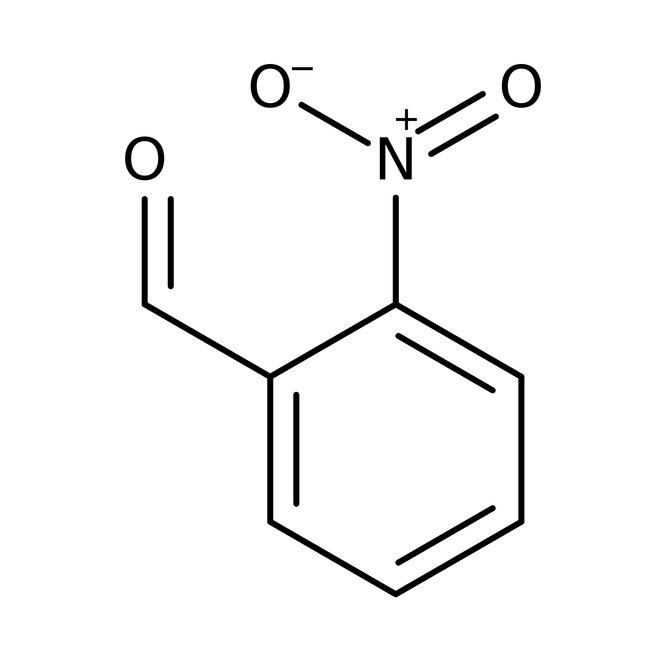
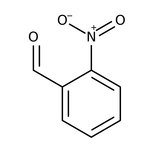
2-Nitrobenzaldehyde, 98+%, Thermo Scientific Chemicals
CAS: 552-89-6 | C7H5NO3 | 151.121 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA11501.14 | 25 g |
Catalog number ALFA11501.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Chemical Identifiers
CAS27607-77-8
IUPAC Nametrimethylsilyl trifluoromethanesulfonate
Molecular FormulaC4H9F3O3SSi
InChI KeyFTVLMFQEYACZNP-UHFFFAOYSA-N
SMILESC[Si](C)(C)OS(=O)(=O)C(F)(F)F
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless to light brown
Titration with NaOH>=98.5 %
Refractive index1.3600 to 1.3620 (20°C, 589 nm)
Appearance (Form)Liquid
Infrared spectrumConforms
2-Nitrobenzaldehyde is used as a starting material in synthetic chemistry. It is used as an intermediate during the synthesis of Indigo dye. It is involved in the preparation of colorants such as Indigo carmine. Further, it serves as a useful photoremovable protecting group as well as utilized in the preparation of o-nitrophenylethylene glycol.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Nitrobenzaldehyde is used as a starting material in synthetic chemistry. It is used as an intermediate during the synthesis of Indigo dye. It is involved in the preparation of colorants such as Indigo carmine. Further, it serves as a useful photoremovable protecting group as well as utilized in the preparation of o-nitrophenylethylene glycol.
Solubility
Insoluble in water.
Notes
Incompatible with strong oxidizing agents and strong bases.
2-Nitrobenzaldehyde is used as a starting material in synthetic chemistry. It is used as an intermediate during the synthesis of Indigo dye. It is involved in the preparation of colorants such as Indigo carmine. Further, it serves as a useful photoremovable protecting group as well as utilized in the preparation of o-nitrophenylethylene glycol.
Solubility
Insoluble in water.
Notes
Incompatible with strong oxidizing agents and strong bases.
RUO – Research Use Only
General References:
- The nitro group can be displaced by nucleophiles. Thus, tert-butanethiol gives 2-tert-butylthiobenzaldehyde, a useful intermediate for thiocoumarins and other sulfur-containing heterocycles: Synthesis, 56 (1978).
- For reduction with FeSO4 to the unstable 2-aminobenzaldehyde, see: Org. Synth. Coll., 3, 56 (1955).
- Cummings, M. M.; Söderberg, B. C. G. Reexamination of the Bromination of 2-Nitrobenzaldehyde with NBS or NaBr-NaIO4 in Sulfuric Acid. Synth. Commun. 2014, 44 (7), 954-958.
- Bouya, H.; Errami, M.; Salghi, R.; Ebenso, E. E.; Zarrouk, A.; Chakir, A.; Hammouti, B. Electrochemical Oxidation of 2-Nitrobenzaldehyde on Boron-Doped Diamond Anodes. Int. J. Electrochem. Sci. 2013, 8, 7468-7478.