Search Thermo Fisher Scientific
Thermo Scientific Chemicals
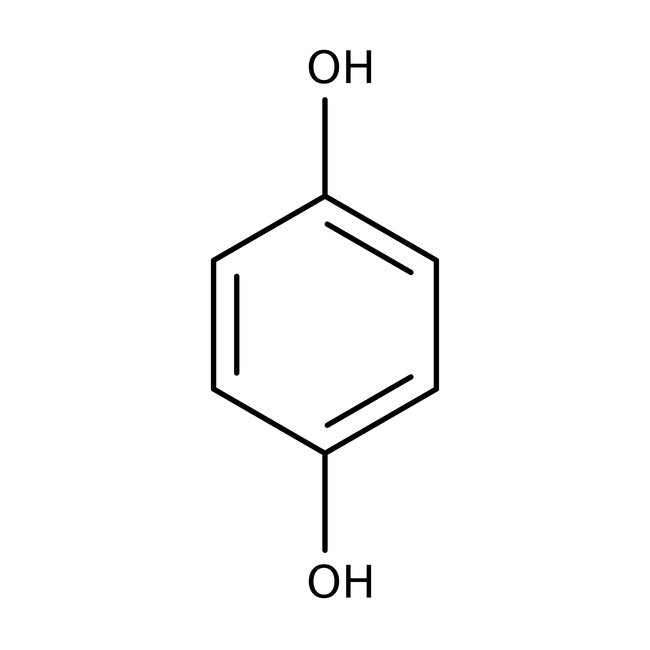
Hydroquinone, 99%, Thermo Scientific Chemicals
CAS: 123-31-9 | C6H6O2 | 110.112 g/mol
Catalog number ALFA11411.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Chemical Identifiers
CAS1122-58-3
IUPAC Name4-(dimethylamino)pyridin-1-ium
Molecular FormulaC7H11N2
InChI KeyVHYFNPMBLIVWCW-UHFFFAOYSA-O
SMILESCN(C)C1=CC=[NH+]C=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Form)Crystalline powder or crystals
Melting point110°C to 113°C
Water=<0.1 %
Appearance (Color)White
Infrared spectrumConforms
View more
Hydroquinone is used as a reducing agent and finds applications in black and white photographic developers for film and paper along with 4-(methylamino)phenol sulfate (metol). In this application, silver halides are reduced to elemental silver. It acts as a polymerization inhibitor which prevents polymerization of acrylic acid, methyl methacrylate, cyanoacrylate and other monomers. It undergoes mild oxidation to convert into parabenzoquinone. It is also used as an intermediate to produce antioxidants for rubber and food.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Hydroquinone is used as a reducing agent and finds applications in black and white photographic developers for film and paper along with 4-(methylamino)phenol sulfate (metol). In this application, silver halides are reduced to elemental silver. It acts as a polymerization inhibitor which prevents polymerization of acrylic acid, methyl methacrylate, cyanoacrylate and other monomers. It undergoes mild oxidation to convert into parabenzoquinone. It is also used as an intermediate to produce antioxidants for rubber and food.
Solubility
Soluble in water, methanol, ethanol, diethyl ether, acetone, benzene and carbon tetrachloride.
Notes
Air and light sensitive. Incompatible with strong bases and strong oxidizing agents.
Hydroquinone is used as a reducing agent and finds applications in black and white photographic developers for film and paper along with 4-(methylamino)phenol sulfate (metol). In this application, silver halides are reduced to elemental silver. It acts as a polymerization inhibitor which prevents polymerization of acrylic acid, methyl methacrylate, cyanoacrylate and other monomers. It undergoes mild oxidation to convert into parabenzoquinone. It is also used as an intermediate to produce antioxidants for rubber and food.
Solubility
Soluble in water, methanol, ethanol, diethyl ether, acetone, benzene and carbon tetrachloride.
Notes
Air and light sensitive. Incompatible with strong bases and strong oxidizing agents.
RUO – Research Use Only
General References:
- Widely used as a radical trap to inhibit polymerization and peroxidation reactions.
- Forms inclusion compounds (clathrates) with three molecules of hydroquinone held together by H bonds to form a cage in which a single guest molecule can be accommodated. For a review, see: D. D. MacNicol in Inclusion Compounds, Vol. 2, J. L. Atwood et al, Eds., Academic Press, London (1984), p1.
- A stable 1:1 complex with hydrazine has been isolated and found to be a useful, safer replacement for anhydrous hydrazine, e.g. in the solid state reaction with esters to give hydrazides: J. Chem. Soc., Chem. Commun., 1531 (1995).
- Boota, M.; Hatzell, K. B.; Kumbur, E. C.; Gogotsi, Y. Towards High-Energy-Density Pseudocapacitive Flowable Electrodes by the Incorporation of Hydroquinone. ChemSusChem 2015, 8 (5), 835-843.
- Buzzo, G. S.; Rodrigues, A. C. B.; De Souza, R. F. B.; Silva, J. C. M.; Bastos, E. L.; Spinace, E. V.; Assumpcao, M. H. M. T. Synthesis of hydroquinone with co-generation of electricity from phenol aqueous solution in a proton exchange membrane fuel cell reactor. Catal. Commun. 2015, 59, 113-115.