Search Thermo Fisher Scientific
Thermo Scientific Chemicals
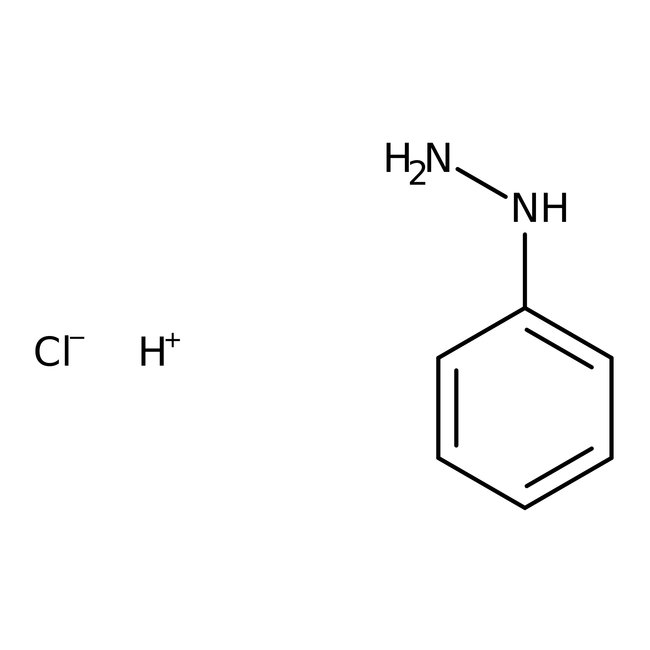
Phenylhydrazine, 97%, Thermo Scientific Chemicals
CAS: 100-63-0 | C6H8N2 | 108.144 g/mol
Catalog number ALFA11246.18
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
50 g
Specifications
Chemical Name or MaterialPhenylhydrazine
CAS100-63-0
Health Hazard 1H227-H301+H311+H331-H315-H317-H319-H335-H341-H350-H372
Health Hazard 2GHS H Statement
H301-H311-H331-H350-H372-H341-H227-H315-H319-H317
H301-H311-H331-H350-H372-H341-H227-H315-H319-H317
Health Hazard 3P201-P202-P210-P235-P260-P264b-P270-P271-P272-P280-P281-P301+P310-P302+P352-P304+P340-P305+P351+P338-P308+P313-P311-P312-P330-P333+P313-P361-P363-P370+P378q-P501c
View more
Phenyl hydrazine is involved in Fischer indole synthesis to prepare indoles, which find application as intermediates in the pharmaceuticals particularly for the tryptamine drug. In analytical chemistry, it is used to differentiate and separate sugars by forming phenyhydrazones. It is used as an N-protecting reagent and for cleavage of phthaloyl group.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Phenyl hydrazine is involved in Fischer indole synthesis to prepare indoles, which find application as intermediates in the pharmaceuticals particularly for the tryptamine drug. In analytical chemistry, it is used to differentiate and separate sugars by forming phenyhydrazones. It is used as an N-protecting reagent and for cleavage of phthaloyl group.
Solubility
Soluble in dilute acids.
Notes
Air and light sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with oxidizing agents.
Phenyl hydrazine is involved in Fischer indole synthesis to prepare indoles, which find application as intermediates in the pharmaceuticals particularly for the tryptamine drug. In analytical chemistry, it is used to differentiate and separate sugars by forming phenyhydrazones. It is used as an N-protecting reagent and for cleavage of phthaloyl group.
Solubility
Soluble in dilute acids.
Notes
Air and light sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Derivatizing agent for the conversion of carbonyl compounds to their phenylhydrazones best known as intermediates in the Fischer indole synthesis, by cyclization under acidic conditions with loss of ammonia. The classical reagent is anhydrous ZnCl2: Org. Synth. Coll., 3, 725 (1976). For reviews, see: Chem. Rev., 63, 373 (1963); 69, 227 (1969); Tetrahedron, 36, 161 (1980); Org. Prep. Proced. Int., 25, 609 (1993). Milder cyclization conditions are sometimes effective, e.g.: dilute H2SO4: J. Org. Chem., 59, 3738 (1994); PCl3: J. Chem. Soc., Chem. Commun., 563 (1981); Tetrahedron, 41, 4615 (1985). Cyclohexanone phenylhydrazone gives tetrahydrocarbazole on heating in acetic acid: Org. Synth. Coll., 4, 884 (1963).
- Methods used for the cleavage of phenylhydrazones include exchange by refluxing with acetone: J. Org. Chem., 40, 3302 (1975), or oxidative cleavage by Fe(NO3)3 on a clay support: Synthesis, 439 (1985).
- Wang, L.; Wang, P.; Liu, Y.; Zheng, L.; Sun, Q.; Qiu, S.; Liu, C. Effects of phenylhydrazine-4-sulfonic acid on the reduction of GO and preparation of hydrophilic graphene with broad pH stability and antioxidant activity. RSC Adv. 2015, 5 (48), 38696-38705.
- Gein, V. L.; Mar’yasov, M. A. Reactions of 5-aryl-4-(hetaren-2-ylcarbonyl)-3-hydroxy-1-(1, 3-thiazol-2-yl)-2, 5-dihydro-1H-pyrrol-2-ones with hydrazine, phenylhydrazine, and hydroxylamine. Russ. J. Org. Chem. 2015, 51 (1), 110-115.