Search Thermo Fisher Scientific
Thermo Scientific Chemicals
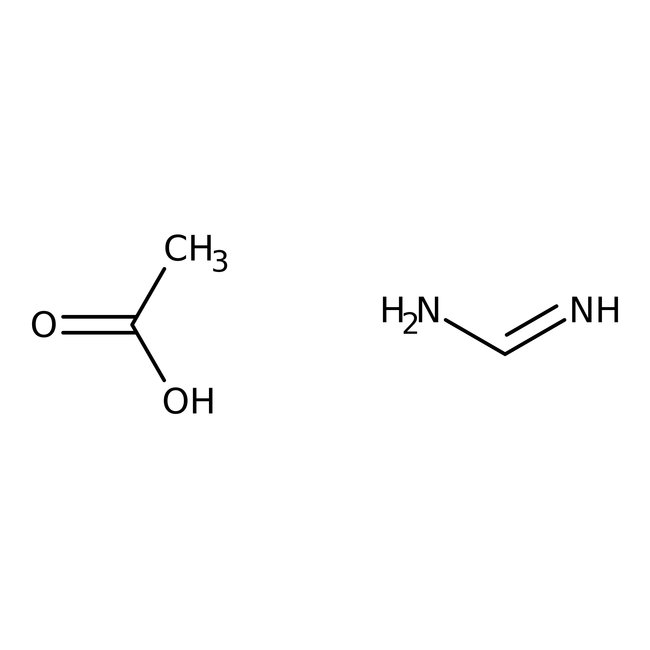
Formamidine acetate, 99%, Thermo Scientific Chemicals
CAS: 3473-63-0 | C3H8N2O2 | 104.109 g/mol
Catalog number ALFA11158.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Chemical Identifiers
CAS10025-69-1
IUPAC Nameλ²-tin(2+) dihydrate dichloride
Molecular FormulaCl2H4O2Sn
InChI KeyFWPIDFUJEMBDLS-UHFFFAOYSA-L
SMILESO.O.[Cl-].[Cl-].[Sn++]
View more
Specifications Specification Sheet
Specification Sheet
FormCrystalline powder or crystals and/or chunks
Identification (FTIR)Conforms
Appearance (Color)White
Assay (Titration ex Chloride)≥97.5 to ≤102.5%
Melting Point (clear melt)41-48?C
Formamidine acetate is used as an intermediate in the synthesis of active pharmaceutical ingredients. It is also used as a condensing agent for the preparation of pyrimidine and imidazole heterocycles.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Formamidine acetate is used as an intermediate in the synthesis of active pharmaceutical ingredients. It is also used as a condensing agent for the preparation of pyrimidine and imidazole heterocycles.
Solubility
Soluble in water and hot alcohol.
Notes
Hygroscopic. Moisture sensitive. Incompatible with oxidizing agents.
Formamidine acetate is used as an intermediate in the synthesis of active pharmaceutical ingredients. It is also used as a condensing agent for the preparation of pyrimidine and imidazole heterocycles.
Solubility
Soluble in water and hot alcohol.
Notes
Hygroscopic. Moisture sensitive. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- More convenient source of formamidine than the corresponding chloride in that it is non-hygroscopic and, being the salt of a weaker acid, can often be used in synthesis without the need to liberate the free base.
- Widely used in heterocyclic syntheses, for example of pyrimidine and imidazole derivatives.
- Maiden, T. M. M.; Swanson, S.; Procopiou, P. A.; Harrity, J. P. A Mild and Regioselective Route to Functionalized Quinazolines. Chem. Eur. J. 2015, 21 (41), 14342-14346.
- Cebrián, C.; Natali, M.; Villa, D.; Panigati, M.; Mauro, M.; D'Alfonso, G.; De Cola, L. Luminescent supramolecular soft nanostructures from amphiphilic dinuclear Re(I) complexes. Nanoscale 2015, 7, 12000-12009.