Search Thermo Fisher Scientific
Thermo Scientific Chemicals
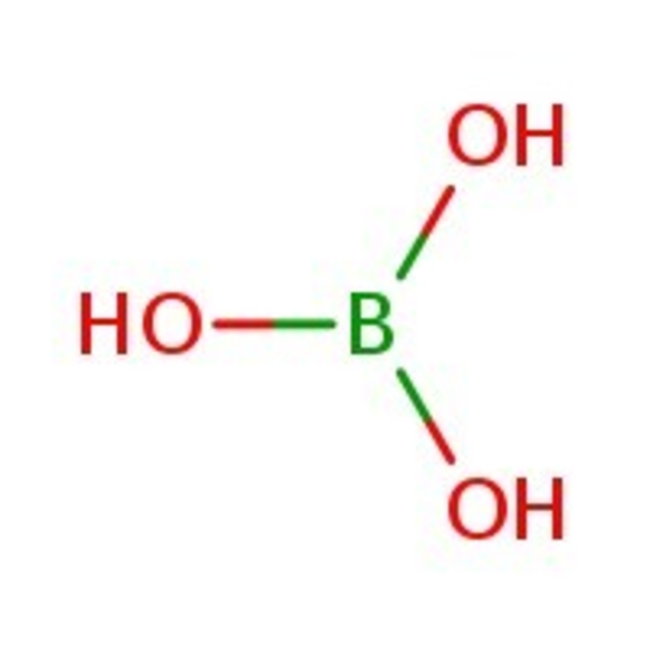
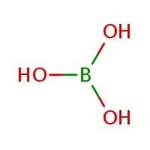
Boric acid, 99+%, Thermo Scientific Chemicals
CAS: 10043-35-3 | BH3O3 | 61.83 g/mol
Catalog number ALFA10896.0E
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
2500 g
Chemical Identifiers
CAS3031-68-3
IUPAC Namehexa-2,4-diyne-1,6-diol
Molecular FormulaC6H6O2
InChI KeyJXMQYKBAZRDVTC-UHFFFAOYSA-N
SMILESOCC#CC#CCO
View more
Specifications Specification Sheet
Specification Sheet
FormPowder
Assay (GC)>96.0%
Appearance (Color)Cream to brown or pale orange
Boric acid is a precursor material for other boron compounds. A dilute water solution of boric acid is usually employed as a mild antiseptic and eyewash. Boric acid is employed in leather manufacture, electroplating and cosmetics. It is involved in the production of monofilament fiberglass which finds applications in boats, industrial piping, LCD flat panel displays and computer circuit boards. It is in combination with sodium tetraborate decahydrate (borax) and is used as a welding flux by blacksmiths. The mixture of boric acid and silicone oil is useful in the production of silly putty.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Boric acid is a precursor material for other boron compounds. A dilute water solution of boric acid is usually employed as a mild antiseptic and eyewash. Boric acid is employed in leather manufacture, electroplating and cosmetics. It is involved in the production of monofilament fiberglass which finds applications in boats, industrial piping, LCD flat panel displays and computer circuit boards. It is in combination with sodium tetraborate decahydrate (borax) and is used as a welding flux by blacksmiths. The mixture of boric acid and silicone oil is useful in the production of silly putty.
Solubility
Soluble in water and alcohols. Slightly soluble in acetone and pyridine.
Notes
Store in cool place. Moisture sensitive. Incompatible with potassium and acid anhydrides.
Boric acid is a precursor material for other boron compounds. A dilute water solution of boric acid is usually employed as a mild antiseptic and eyewash. Boric acid is employed in leather manufacture, electroplating and cosmetics. It is involved in the production of monofilament fiberglass which finds applications in boats, industrial piping, LCD flat panel displays and computer circuit boards. It is in combination with sodium tetraborate decahydrate (borax) and is used as a welding flux by blacksmiths. The mixture of boric acid and silicone oil is useful in the production of silly putty.
Solubility
Soluble in water and alcohols. Slightly soluble in acetone and pyridine.
Notes
Store in cool place. Moisture sensitive. Incompatible with potassium and acid anhydrides.
RUO – Research Use Only
General References:
- Promotes the decarboalkoxylation of malonic esters and analogous compounds: Synth. Commun., 9, 609 (1979); Org. Synth. Coll., 6, 615 (1988); Can. J. Chem., 69, 1201 (1991).
- Catalyzes the N-acylation of indoles with carboxylic acids: Heterocycles, 19, 91 (1982). Promotes the reaction between Benzenesulfinic acid sodium salt, A12118, and propiolate esters, under phase-transfer conditions, to give (Z)-vinyl sulfones: Org. Synth. Coll., 8, 458 (1993).
- Matsumiya, H.; Hara, T. Conversion of glucose into 5-hydroxymethylfurfural with boric acid in molten mixtures of choline salts and carboxylic acids. Biomass Bioenergy 2015, 72, 227-232.
- Miyazaki, T.; Takeda, Y.; Hoshiko, A.; Shimokita, K.; Ogomi, D. Evaluation of oriented amorphous regions in polymer films during uniaxial deformation; structural characterization of a poly(vinyl alcohol) film during stretching in boric acid aqueous solutions. Polym. Eng. Sci. 2015, 55 (3), 513-522.