Search Thermo Fisher Scientific
Thermo Scientific Chemicals
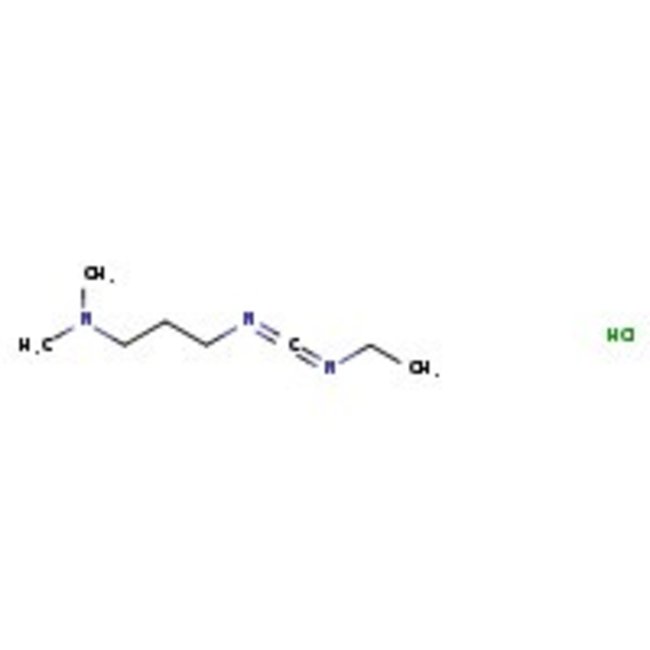
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, 98+%, Thermo Scientific Chemicals
CAS: 25952-53-8 | C8H18ClN3 | 191.70 g/mol
Catalog number ALFA10807.14
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
25 g
Chemical Identifiers
CAS74163-81-8
Specifications Specification Sheet
Specification Sheet
FormPowder
Infrared spectrumConforms
Appearance (Color)White to pale cream
Assay from Supplier's CofA≥96.0%
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride is used as a carboxyl activating agent. It is used in peptide synthesis, 3'-amino-3'-deoxyadenosine-5'-di- and triphosphates and in the preparation of antibodies like immunoconjugates. It plays a vital role for immobilization of large biomolecules in association with N-hydroxysuccinimide. It is also used in the acylation of phosphoranes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride is used as a carboxyl activating agent. It is used in peptide synthesis, 3′-amino-3′-deoxyadenosine-5′-di- and triphosphates and in the preparation of antibodies like immunoconjugates. It plays a vital role for immobilization of large biomolecules in association with N-hydroxysuccinimide. It is also used in the acylation of phosphoranes.
Solubility
Soluble in acetonitrile dichloromethane, dimethylformamide, and tetrahydrofuran. Slightly soluble in water.
Notes
Sensitive to moisture. Incompatible with strong acids, strong oxidizing agents and moisture.
1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride is used as a carboxyl activating agent. It is used in peptide synthesis, 3′-amino-3′-deoxyadenosine-5′-di- and triphosphates and in the preparation of antibodies like immunoconjugates. It plays a vital role for immobilization of large biomolecules in association with N-hydroxysuccinimide. It is also used in the acylation of phosphoranes.
Solubility
Soluble in acetonitrile dichloromethane, dimethylformamide, and tetrahydrofuran. Slightly soluble in water.
Notes
Sensitive to moisture. Incompatible with strong acids, strong oxidizing agents and moisture.
RUO – Research Use Only
General References:
- Water-soluble carbodiimide, widely used for peptide coupling (see Appendix 6), with the major advantage that excess reagent and the urea by-product can be easily removed by washing with dilute acid or water: J. Org. Chem., 26, 2525 (1961); J. Am. Chem. Soc., 87, 2492 (1965). For discussion of the mechanism of peptide coupling with this reagent, see: J. Am. Chem. Soc., 103, 7090 (1981).
- Often used in conjunction with an additive such as HOBt (1-hydroxybenzotriazole hydrate) to suppress racemization; see, for example: Bull. Chem. Soc. Jpn., 55, 2165 (1982). A two-phase solvent system (dichloromethane-water or isopropyl acetate-water) has been found to give superior results in this type of peptide coupling: J. Org. Chem., 60, 3569 (1995). In a comparative study, submolar quantities HOBt in DMF-water gave good results: Chem. Lett., 1 (1997).
- For use in the synthesis of cyclic derivatives of 3'-amino-3'-deoxyadenosine-5'-di- and triphosphates, see: Angew. Chem. Int. Ed., 33, 1394 (1994).
- Promotes various other N-acylation reactions such as the formation of N-acylsulfonamides from primary sulfonamides and N-protected amino acids in the presence of DMAP: Synlett, 1141 (1995).
- For use in the acylation of phosphoranes, see (Cyanomethyl) triphenyl phosphonium chloride, A13096.
- Liu, J. A.; Guo, X. P.; Liang, S.; An, F.; Shen, H. Y.; Xu, Y. J. Regioselective synthesis of 5'-amino acid esters of some nucleosides via orthogonal protecting protocol. Tetrahedron 2015, 71 (9), 1409-1412.
- Meng, H.; Zheng, J.; Wen, X.; Cai, Z.; Zhang, J.; Chen, T. pH- and Sugar-Induced Shape Memory Hydrogel Based on Reversible Phenylboronic Acid-Diol Ester Bonds. Macromol. Rapid Commun. 2015, 36 (6), 533-537.