Search Thermo Fisher Scientific
Thermo Scientific Chemicals
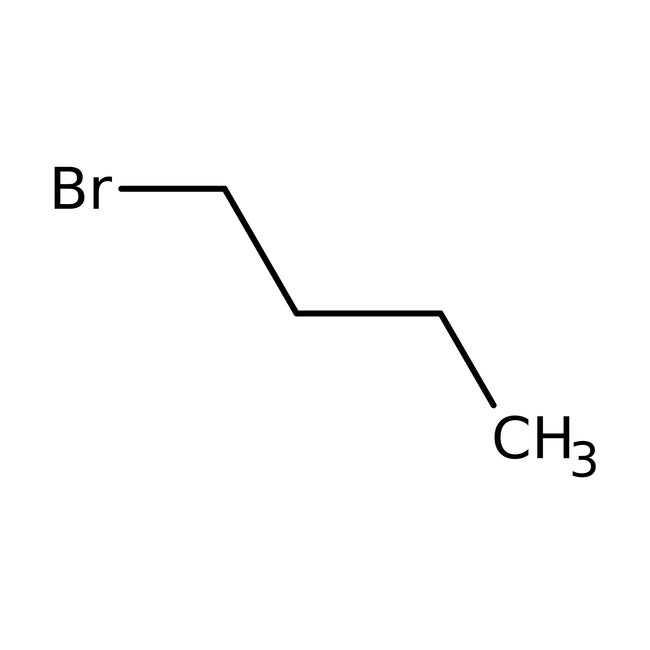
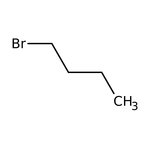
1-Bromobutane, 98+%, Thermo Scientific Chemicals
CAS: 109-65-9 | C4H9Br | 137.02 g/mol
Catalog number ALFA10696.36
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
500 g
Specifications
Chemical Name or Material1-Bromobutane
CAS109-65-9
Health Hazard 1H225-H315-H319
Health Hazard 2GHS H Statement
H225-H315-H319-H335
H225-H315-H319-H335
Health Hazard 3P210-P233-P240-P241-P242-P243-P264b-P280-P303+P361+P353-P305+P351+P338-P332+P313-P363-P370+P378q-P501c
View more
1-Bromobutane is used as an intermediate in organic synthesis and as a solvent for cleaning and degreasing. It acts as an alkylating agent as well as to prepare organometallic compounds such as n-butyllithium. It is also involved in the synthesis of procaine and tetracaine. It reacts with magnesium metal to prepare the Grignard reagent, which is used to form carbon-carbon bonds.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-Bromobutane is used as an intermediate in organic synthesis and as a solvent for cleaning and degreasing. It acts as an alkylating agent as well as to prepare organometallic compounds such as n-butyllithium. It is also involved in the synthesis of procaine and tetracaine. It reacts with magnesium metal to prepare the Grignard reagent, which is used to form carbon-carbon bonds.
Solubility
Miscible with ethanol and diethyl ether. Immiscible with water.
Notes
Incompatible with strong oxidizing agents, strong bases, magnesium, potassium, sodium and sodium oxides.
1-Bromobutane is used as an intermediate in organic synthesis and as a solvent for cleaning and degreasing. It acts as an alkylating agent as well as to prepare organometallic compounds such as n-butyllithium. It is also involved in the synthesis of procaine and tetracaine. It reacts with magnesium metal to prepare the Grignard reagent, which is used to form carbon-carbon bonds.
Solubility
Miscible with ethanol and diethyl ether. Immiscible with water.
Notes
Incompatible with strong oxidizing agents, strong bases, magnesium, potassium, sodium and sodium oxides.
RUO – Research Use Only
General References:
- Chief Elk, J.; Benjamin, I. beta-Cyclodextrin at the water/1-bromobutane interface: molecular insight into reverse phase transfer catalysis. Langmuir 2015, 31 (18), 5086-5092.
- Paulechka, Y. U.; Kabo, G. J.; Blokhin, A. V.; Firaha, D. S. Thermodynamics of ionic liquid precursors. 1-Bromobutane and its isomers. J. Chem. Eng. Data 2011, 56 (12), 4891-4899.