Search Thermo Fisher Scientific
Thermo Scientific Chemicals
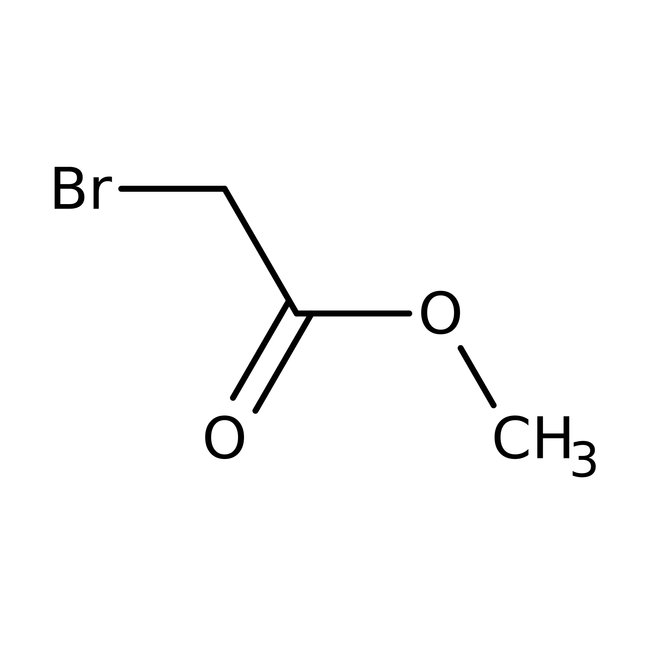
Methyl bromoacetate, 98+%, Thermo Scientific Chemicals
CAS: 96-32-2 | C3H5BrO2 | 152.975 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA10605.30 | 250 g |
Catalog number ALFA10605.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Specifications
Chemical Name or MaterialMethyl bromoacetate
CAS96-32-2
Health Hazard 1H227-H301-H314-H317-H335
Health Hazard 2GHS H Statement
H301-H311-H331-H314-H318-H227
Toxic if swallowed.
Toxic in contact with skin.
Toxic if inhaled.
Causes severe skin burns and eye damage.
Causes serious eye damage.
Combustible liquid.
H301-H311-H331-H314-H318-H227
Toxic if swallowed.
Toxic in contact with skin.
Toxic if inhaled.
Causes severe skin burns and eye damage.
Causes serious eye damage.
Combustible liquid.
Health Hazard 3P210-P235-P260-P264b-P270-P271-P272-P280-P303+P361+P353-P304+P340-P305+P351+P338-P310-P330-P331-P333+P313-P363-P370+P378q-P501c
View more
Methyl bromoacetate is used in the synthesis of coumarins and cis-cyclopropanes. It reacts with the conjugate base of (methylmethoxycarbene)pentacarbonylchromium(0) to prepare alkylated carbene complexes. Further, it is used to make vitamins and pharmaceuticals.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Methyl bromoacetate is used in the synthesis of coumarins and cis-cyclopropanes. It reacts with the conjugate base of (methylmethoxycarbene)pentacarbonylchromium(0) to prepare alkylated carbene complexes. Further, it is used to make vitamins and pharmaceuticals.
Solubility
Miscible with methanol, ether and acetone. Slightly miscible with water
Notes
Incompatible with acids, bases, oxidizing agents and reducing agents.
Methyl bromoacetate is used in the synthesis of coumarins and cis-cyclopropanes. It reacts with the conjugate base of (methylmethoxycarbene)pentacarbonylchromium(0) to prepare alkylated carbene complexes. Further, it is used to make vitamins and pharmaceuticals.
Solubility
Miscible with methanol, ether and acetone. Slightly miscible with water
Notes
Incompatible with acids, bases, oxidizing agents and reducing agents.
RUO – Research Use Only
General References:
- Dib, J.; Schlörer, N.; Schänzer, W.; Thevis, M. Studies on the collision-induced dissociation of adipoR agonists after electrospray ionization and their implementation in sports drug testing. J. Mass Spectrom. 2015, 50 (2), 407-417.
- Hoashi, Y.; Takai, T.; Koike, T.; Uchikawa, O. Synthesis of a novel tricyclic 1, 6, 7, 8-tetrahydro-2H-cyclopenta [b] furo [3, 2-d] pyridine derivative, the 5-aza analog of ramelteon. Tetrahedron Lett. 2014, 55 (29), 4014-4016.