Search Thermo Fisher Scientific
Thermo Scientific Chemicals
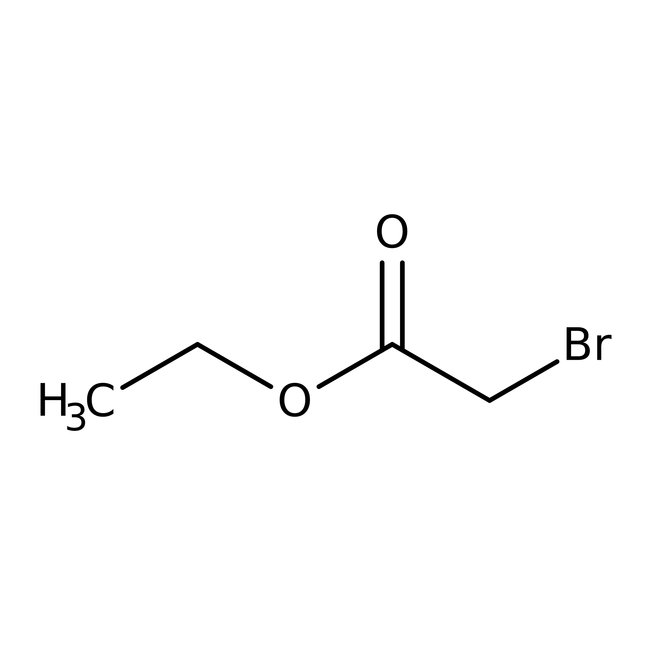
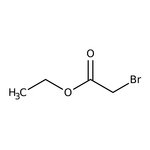
Ethyl bromoacetate, 98%, Thermo Scientific Chemicals
CAS: 105-36-2 | C4H7BrO2 | 167.002 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFA10448.22 | 100 g |
Catalog number ALFA10448.22
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
100 g
Chemical Identifiers
CAS7440-16-6
IUPAC Namerhodium
Molecular FormulaRh
InChI KeyMHOVAHRLVXNVSD-UHFFFAOYSA-N
SMILES[Rh]
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Grey-black to black
Assay4.5 to 5.5 % (Rh)
Particle Size/Sieve analysis< 8 um: 21%
Particle Size/Sieve analysis<128 um: 99%
Particle Size/Sieve analysis< 16 um: 39%
View more
Ethyl bromoacetate is widely used as an alkylating reagent involved in the Reformatsky reaction to prepare the beta-hydroxy esters by reacting with carbonyl compounds. It is also used for the syntheses of witting reagent, artificial diethylstilbestrol antigen, 3-phenyl-1-naphthol and steroidal thiazolidinone derivatives. It finds applications in the preparation of reversibly photoresponsive coumarin-stabilized polymeric nanoparticles in an aqueous medium which act as a detectable drug carrier.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Ethyl bromoacetate is widely used as an alkylating reagent involved in the Reformatsky reaction to prepare the beta-hydroxy esters by reacting with carbonyl compounds. It is also used for the syntheses of witting reagent, artificial diethylstilbestrol antigen, 3-phenyl-1-naphthol and steroidal thiazolidinone derivatives. It finds applications in the preparation of reversibly photoresponsive coumarin-stabilized polymeric nanoparticles in an aqueous medium which act as a detectable drug carrier.
Solubility
Miscible with ethanol, acetone, benzene and ethyl ether. Immiscible with water.
Notes
It is a lachrymator. Incompatible with strong oxidizing agents.
Ethyl bromoacetate is widely used as an alkylating reagent involved in the Reformatsky reaction to prepare the beta-hydroxy esters by reacting with carbonyl compounds. It is also used for the syntheses of witting reagent, artificial diethylstilbestrol antigen, 3-phenyl-1-naphthol and steroidal thiazolidinone derivatives. It finds applications in the preparation of reversibly photoresponsive coumarin-stabilized polymeric nanoparticles in an aqueous medium which act as a detectable drug carrier.
Solubility
Miscible with ethanol, acetone, benzene and ethyl ether. Immiscible with water.
Notes
It is a lachrymator. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Zhang, Y.; Zhao, K. T.; Fox, S. G.; Kim, J.; Kirsch, D. R.; Ferrante, R. J.; Silverman, R. B. Tertiary amine pyrazolones and their salts as inhibitors of mutant superoxide dismutase 1-dependent protein aggregation for the treatment of amyotrophic lateral sclerosis. J. Med. Chem. 2015, 58 (15), 5942-5949.
- Grzybowski, M., & Gryko, D. T. Diketopyrrolopyrroles: Synthesis, Reactivity, and Optical Properties. Adv. Opt. Mater. 2015, 3 (3), 280-320.