Search Thermo Fisher Scientific
Thermo Scientific Chemicals
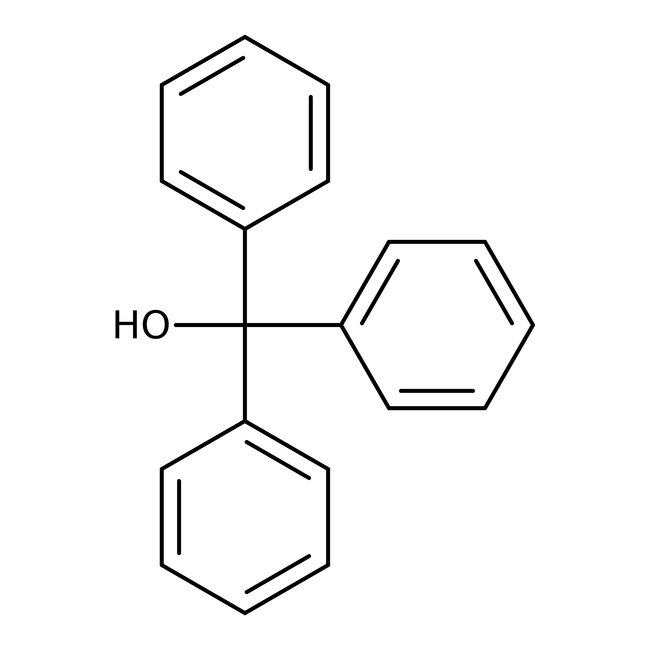
Triphenylmethanol, 98%, Thermo Scientific Chemicals
CAS: 76-84-6 | C19H16O | 260.34 g/mol
Catalog number ALFA10366.30
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
250 g
Specifications
Chemical Name or MaterialTriphenylmethanol
CAS76-84-6
Health Hazard 1Warning
Health Hazard 2GHS H Statement
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
H315-H319-H335
Causes skin irritation.
Causes serious eye irritation.
May cause respiratory irritation.
Health Hazard 3GHS P Statement
P261-P280-P305+P351+P338-P304+P340-P405-P501a
Avoid breathing dust/fume/gas/mist/vapors/spray.
Wear protective gloves/protective clothing/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do.
Continue rinsing.
IF INHALED: Remove to fresh air and keep at rest in a position comfortable for breathing.
Store locked up.
Dispose of contents/container in accordance with local/regional/national/international regulations.
P261-P280-P305+P351+P338-P304+P340-P405-P501a
Avoid breathing dust/fume/gas/mist/vapors/spray.
Wear protective gloves/protective clothing/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do.
Continue rinsing.
IF INHALED: Remove to fresh air and keep at rest in a position comfortable for breathing.
Store locked up.
Dispose of contents/container in accordance with local/regional/national/international regulations.
View more
Triphenylmethanol is used as a reagent in the research laboratory. It acts as an intermediate in the production of the commercially useful triarylmethane dyes. It is used in the preparation of triphenylmethane. It is also used as an antiproliferative agent. Further, it is used in the preparation of two-electron reduction product of pyrylogen. In addition to this, it reacts with triphenylphosphine oxide to form a 1:1 molecular complex. It serves as a specific clathrate host for methanol and dimethyl sulphoxide and forms clathrate inclusion complexes.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Triphenylmethanol is used as a reagent in the research laboratory. It acts as an intermediate in the production of the commercially useful triarylmethane dyes. It is used in the preparation of triphenylmethane. It is also used as an antiproliferative agent. Further, it is used in the preparation of two-electron reduction product of pyrylogen. In addition to this, it reacts with triphenylphosphine oxide to form a 1:1 molecular complex. It serves as a specific clathrate host for methanol and dimethyl sulphoxide and forms clathrate inclusion complexes.
Solubility
Soluble in dioxane, ethanol, water, ether and benzene.
Notes
Incompatible with acids, acid chlorides, acid anhydrides and oxidizing agents.
Triphenylmethanol is used as a reagent in the research laboratory. It acts as an intermediate in the production of the commercially useful triarylmethane dyes. It is used in the preparation of triphenylmethane. It is also used as an antiproliferative agent. Further, it is used in the preparation of two-electron reduction product of pyrylogen. In addition to this, it reacts with triphenylphosphine oxide to form a 1:1 molecular complex. It serves as a specific clathrate host for methanol and dimethyl sulphoxide and forms clathrate inclusion complexes.
Solubility
Soluble in dioxane, ethanol, water, ether and benzene.
Notes
Incompatible with acids, acid chlorides, acid anhydrides and oxidizing agents.
RUO – Research Use Only
General References:
- Protection of thiols, such as cysteine residues in peptide synthesis, as their S-trityl derivatives can be accomplished in high yield in TFA. Cleavage can be effected with HBr in AcOH: J. Chem. Soc. (C), 2683 (1970), with Hg(II) salts, or by oxidation to the disulfide with I2 in MeOH: Helv. Chim. Acta, 51, 2061 (1968). See also Appendix 6.
- Konshin, V.; Turmasova, A.; Konshina, D. Lewis Acid Catalyzed Reaction of Triphenylmethanol with Acetylacetone. Lett. Org. Chem. 2015, 12 (7), 511-515.
- Drożdż, W.; Kołodziejski, M.; Markiewicz, G.; Jenczak, A.; Stefankiewicz, A. R. Generation of a Multicomponent Library of Disulfide Donor-Acceptor Architectures Using Dynamic Combinatorial Chemistry. Int. J. Mol. Sci. 2015, 16 (7), 16300-16312.