Search Thermo Fisher Scientific
Thermo Scientific Chemicals
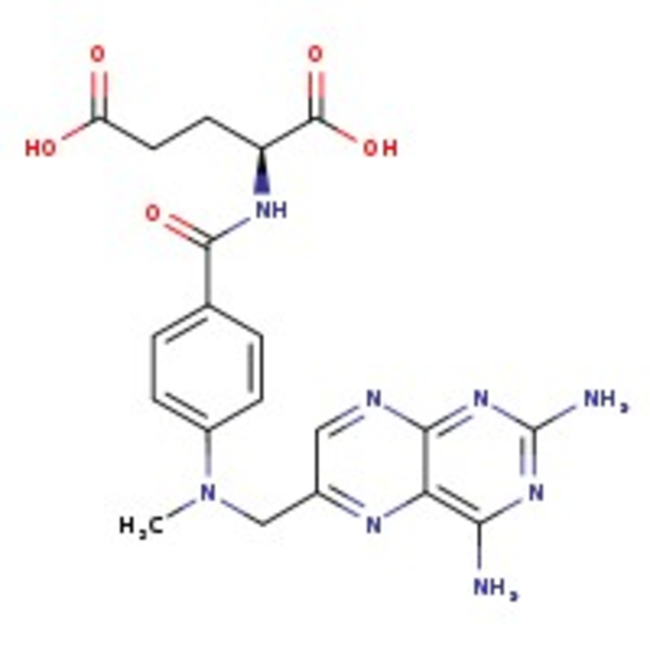
L(+)-Amethopterin hydrate, 99%, Thermo Scientific Chemicals
L(+)-Amethopterin hydrate, CAS # 133073-73-1, is an effective inhibitor of dihydrofolate reductase, which may have anti-tumor activity.
Catalog number FSA208101000
Price (MYR)
586.00
Quantity:
100 mg
Packaging:
Glass Bottle
Price (MYR)
586.00
Chemical Identifiers
CAS389-08-2
IUPAC Name1-ethyl-7-methyl-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid
Molecular FormulaC12H12N2O3
InChI KeyMHWLWQUZZRMNGJ-UHFFFAOYSA-N
SMILESCCN1C=C(C(O)=O)C(=O)C2=CC=C(C)N=C12
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to light yellow
Titration with NaOH>=99.4 %
Appearance (Form)Powder
Melting point225°C to 231°C
Infrared spectrumConforms
This Thermo Scientific Chemicals brand product was originally part of the Acros Organics product portfolio. Some documentation and label information may refer to the legacy brand. The original Acros Organics product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
General Description
- L(+)-Amethopterin hydrate, is a potent inhibitor of dihydrofolate reductase
- It has tumoricidal activity
Applications
- L(+)-Amethopterin hydrate is an inhibitor of dihydrofolate reductase
- It can be used in anti-tumor studies
- It is a cell cycle arrest agent, which can inhibit synthesis of DNA, RNA, and proteins
- It can also inhibit production of pro-inflammatory cytokines
RUO – Research Use Only
General References:
- Friedman, B.; Cronstein, B. Methotrexate mechanism in treatment of rheumatoid arthritis. Joint Bone Spine. 2019, 86(3), 301-307.
- Sasso, S.P.; Gilli, R.M.; Sari, J.C.; Rimet, O.S.; Briand, C.M. Thermodynamic study of dihydrofolate reductase inhibitor selectivity. Biochim Biophys Acta. 1994, 20, 1207(1), 74-9.
- Pittman, S.M.; Strickland, D.; Ireland, C.M. Polymerization of tubulin in apoptotic cells is not cell cycle dependent. Exp Cell Res. 1994, 215 (2), 263-72.