Search Thermo Fisher Scientific
Thermo Scientific Chemicals
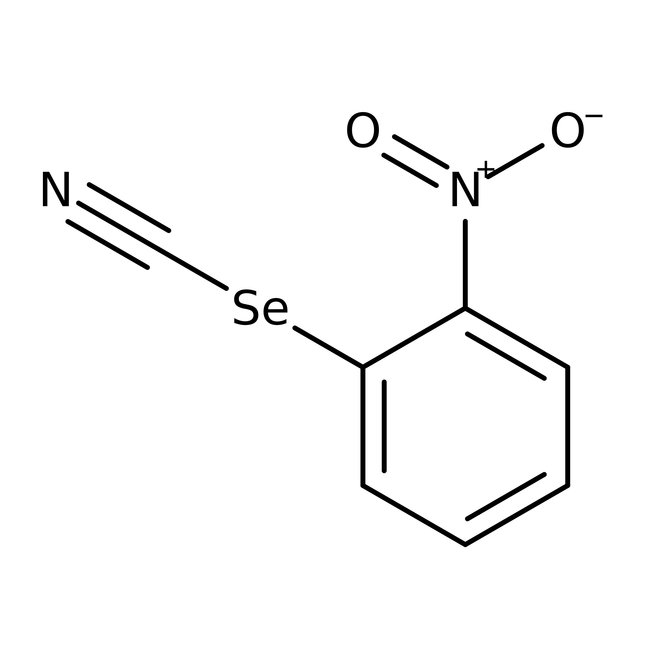
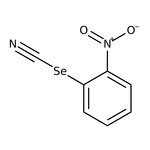
2-Nitrophenylselenocyanate, 97%, Thermo Scientific Chemicals
CAS: 51694-22-5 | C7H4N2O2Se | 227.092 g/mol
| Catalog Number | Quantity |
|---|---|
| ALFL20193.03 | 1 g |
Catalog number ALFL20193.03
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
1 g
Chemical Identifiers
CAS20989-17-7
IUPAC Name(2S)-2-amino-2-phenylethan-1-ol
Molecular FormulaC8H11NO
InChI KeyIJXJGQCXFSSHNL-MRVPVSSYSA-N
SMILESN[C@H](CO)C1=CC=CC=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to light yellow
Melting point75°C to 79°C
HPLC>=97.5 %
Appearance (Form)Crystalline powder
Infrared spectrumConforms
View more
2-Nitrophenylselenocyanate is used to study the mechanism of its reaction with the zinc/thiolate clusters of metallothionein. It is also used in the preparation of 2,3-seco-5 alfa-cholestane-2,3-diol and in the preparation of 4alfa-methyl-2,3-seco-5 alfa-cholestane-2,3-diol. Further, it is used in the preparation of 2-nitrophenylselenyl derivative and 3alfa-[(2-Nitrophenyl)seleno]androsta-1,5-dien-17beta-ol. In addition to this, it reacts with 6-bromo-hexanoic acid methyl ester to get 6-(2-nitro-phenylselanyl)-hexanoic acid methyl ester.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
2-Nitrophenylselenocyanate is used to study the mechanism of its reaction with the zinc/thiolate clusters of metallothionein. It is also used in the preparation of 2,3-seco-5 alfa-cholestane-2,3-diol and in the preparation of 4alfa-methyl-2,3-seco-5 alfa-cholestane-2,3-diol. Further, it is used in the preparation of 2-nitrophenylselenyl derivative and 3alfa-[(2-Nitrophenyl)seleno]androsta-1,5-dien-17beta-ol. In addition to this, it reacts with 6-bromo-hexanoic acid methyl ester to get 6-(2-nitro-phenylselanyl)-hexanoic acid methyl ester.
Solubility
Soluble in dichloromethane, tetrahydrofuran, dimethyl formamide and ethanol.
Notes
Incompatible with acids and strong oxidizing agents.
2-Nitrophenylselenocyanate is used to study the mechanism of its reaction with the zinc/thiolate clusters of metallothionein. It is also used in the preparation of 2,3-seco-5 alfa-cholestane-2,3-diol and in the preparation of 4alfa-methyl-2,3-seco-5 alfa-cholestane-2,3-diol. Further, it is used in the preparation of 2-nitrophenylselenyl derivative and 3alfa-[(2-Nitrophenyl)seleno]androsta-1,5-dien-17beta-ol. In addition to this, it reacts with 6-bromo-hexanoic acid methyl ester to get 6-(2-nitro-phenylselanyl)-hexanoic acid methyl ester.
Solubility
Soluble in dichloromethane, tetrahydrofuran, dimethyl formamide and ethanol.
Notes
Incompatible with acids and strong oxidizing agents.
RUO – Research Use Only
General References:
- Kashinath, K.; Dhara, S.; Reddy, D. S. Breaking and Making of Olefins Simultaneously Using Ozonolysis: Application to the Synthesis of Useful Building Blocks and Macrocyclic Core of Solomonamides. Org. Lett. 2015, 17 (9), 2090-2093.
- Bender, M.; Schmidtmann, M.; Summons, R. E.; Rullkötter, J.; Christoffers, J. A Geomimetic Approach to the Formation and Identification of Fossil Sterane Biomarkers in Crude Oil: 18-nor-D-homo-Androstane and 5 alpha,14 beta-Androstane. Chem. Eur. J. 2015, 21 (35), 12501-12508.