Search Thermo Fisher Scientific
Thermo Scientific Chemicals
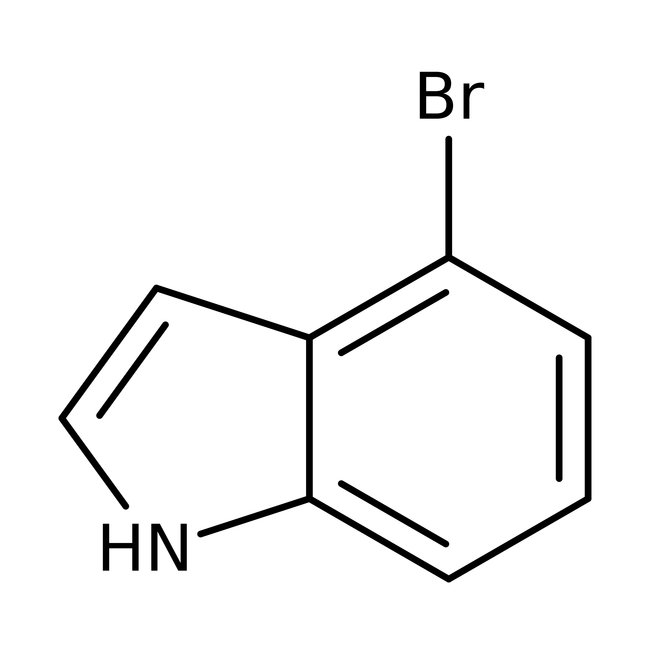
4-Bromoindole, 98%, Thermo Scientific Chemicals
Catalog number ALFL19417.03
View Price:Sign InSign in to see your account pricing. Need an account? Register with us today.
Quantity:
1 g
Specifications
Chemical Name or Material4-Bromoindole
CAS52488-36-5
Health Hazard 1H302+H312+H332-H315-H319-H335
Health Hazard 3P261-P264b-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P312-P330-P332+P313-P362-P501c
Melting Point17°C
View more
4-Bromoindole is a potential inhibitor of GSK-3. It is also used as pharmaceutical intermediates.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
4-Bromoindole is a potential inhibitor of GSK-3. It is also used as pharmaceutical intermediates.
Solubility
Not miscible in water.
Notes
Air & Light Sensitive. Store at 4°C. Store in the dark. Store under dry inert gas. Protect from heat. Incompatible with heat. light, air.
4-Bromoindole is a potential inhibitor of GSK-3. It is also used as pharmaceutical intermediates.
Solubility
Not miscible in water.
Notes
Air & Light Sensitive. Store at 4°C. Store in the dark. Store under dry inert gas. Protect from heat. Incompatible with heat. light, air.
RUO – Research Use Only
General References:
- Yuusaku Yokoyama; Hidemasa Hikawa; Masaharu Mitsuhashi; Aki Uyama; Yasuoki Murakami. Syntheses without protection: a three-step synthesis of optically active clavicipitic acid by utilizing biomimetic synthesis of 4-bromotryptophan. Tetrahedron Letters.1999, 40, 7803-7806.
- Mark C Bagley; Christopher J Moody; Adrian G Pepper. Studies towards the synthesis of diazonamide A. Synthesis of the 4-(oxazol-5-ylmethyl) aryltryptamine fragment. Tetrahedron Letters.2000, 41, 6901-6904.